EU
Label, Labelling vs Instructions for Use (IFU)? 1. A Label is the written, printed, or graphic information that goes on the packaging of the medical device. 2. Instructions For Use (IFUs) or Package Insert is the essential information accompanying the medical device for its safe and effective use by the user. It can be a single to multiple-page document. 3. Labelling is the content that goes on the Label or IFUs. What are the minimum requirements for labeling? The ISO has published many standard...
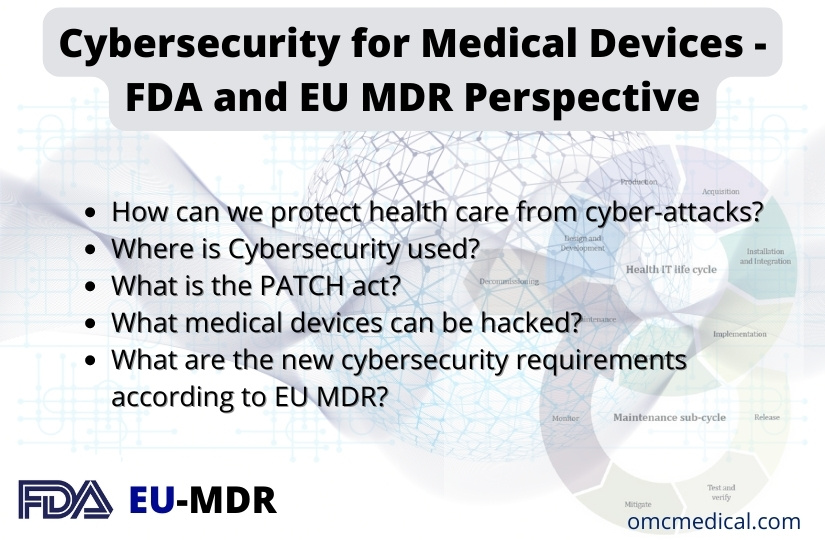
Cybersecurity for Medical Devices – FDA and EU MDR Perspective
Cybersecurity for Medical Devices FDA –Food and Drug Administration The revolution in the digital sector has resulted in the Internet of Things (IoT), Software as a Medical Device (SaMD), Internet of Medical Things (IoMT) and other connected devices permeating the healthcare environment, both in hospital and home, has ended up with the possibility of cyberattacks and intrusions against the connected medical devices and the networks to which such a device is connected. Most Medical devices are...
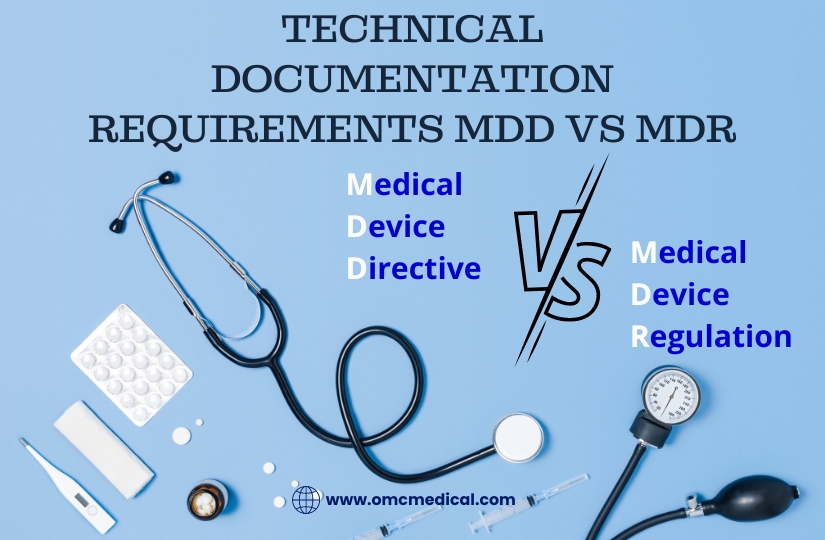
Technical Documentation Requirements MDD Vs MDR
Check out the difference between MDD vs MDR MDD vs MDR MDD A MDD Technical documentation must include: MDR All Technical documentation requirements of MDD must be presented for the MDR alongside the below additional list: Device Description and Specifications Information to be supplied by the manufacturer Design and Manufacturing Information General Safety and Performance Requirements Benefit-Risk Analysis and Risk Management The benefit-risk analysis, the solutions adopted, and the results of t...
Understanding Borderline Cosmetics in the EU
by OMC Medical | Dec 13, 2023 | Cosmetics, EU Understanding the distinctions between product classes within the European cosmetics landscape can be complex and ambiguous, often requiring input from authoritative bodies. Certain products may share similarities but fall under different regulatory frameworks. When products straddle these frameworks, they’re termed “borderline” by the European Commission (EC). Clear comprehension of a product category is vita...
SaMD Devices Classification
SaMD is software intended for one or more medical purposes that perform these without being part of a hardware medical device. Medical device software is meant to be used, alone or in combination, for a purpose specified in the definition of a “medical device” in the MDR or IVDR, regardless of whether the software is independent or driving or influencing the use of a device. You can read more on the SaMD Regulation here. To be qualified as medical device software, the product must first...
Software As a Medical Device and Its Clinical Evaluation
As technology advances across all healthcare fields, Software plays a significant role in all products. It is widely integrated into digital platforms serving both medical and non-medical purposes. Medical device software is one of three types of Software related to medical devices. The other two types of medical device software include Software that is an integral part of the medical device (medical device software) and Software used in manufacturing or maintaining the medical device. Software...
Article 61 Clinical Evaluation in the EU MDR
Article 61 Clinical Evaluation The MDR reinforces the clinical data and evaluation process (article 61 and Annex XIV), and the manufacturer must confirm the device’s conformity to fundamental health and safety requirements using reliable clinical data and evaluation. The clinical evaluation establishes the device’s safety and capacity to fulfil its intended function. It also evaluates adverse side effects and determines whether the benefit-risk ratio is acceptable. Manufacturers must...
Summary of Safety and Clinical Performance (SSCP)
Summary of Safety and Clinical Performance (SSCP) acts as a vital document that allows the public to access information quickly. The information in the SSCP can be sourced entirely from the technical file. The technical file consists of the Post Market Surveillance (PMS), risk assessment, post-market clinical follow-up (PMCF) plans and reports. The SSCP document is required for high-risk devices only-this includes Class III and all implantable devices. Manufacturers of custom-made or investigati...
7 SaMD Device Regulation (UK & Europe)
Under both the EU MDR and the EU IVDR, the MDCG rules specify the conditions for a product to qualify as medical device software (MDSW). An MDSW product can enter the market in one of the ways: 1. As a standalone medical device or 2. As a component of another medical device regulation. The former demands a lengthy regulatory process that includes a conformity assessment to determine whether the medical device meets EU MDR requirements. However, that rule does not apply to products in...
Exceptional Use Medical Devices in the EU and UK
Medical devices conforming with the medical device regulations must have a conformity marking. Medical devices that do not conform may still be placed in the market, provided they apply through the exceptional use devices pathway. This article discusses the exceptional use requirements to be satisfied to gain access to EU and UK markets. In the UK, the UKCA marking displays that the device conforms to UK Medical Device Regulations 2002. Without UKCA marking, the devices can only be placed in the...