EU MDR
Introduction and Guide to Market Access in Europe, Switzerland, and the UK In the intricate landscape of medical device regulations, successfully bringing products to market requires a thorough understanding of the varying requirements in different regions. This market access guide provides comprehensive insights into the European Union (EU), Switzerland, and the United Kingdom (UK) regulations, shedding light on crucial aspects such as Authorized Representatives, Importers, and other economic o...
EU MDR Unveiled: What You Need to Know About Medical Device Regulation in Europe
Introduction about Medical Device Regulation in Europe Introducing the Medical Device Regulation in Europe addresses several challenges. It aims to enhance medical device regulation in Europe to ensure a higher level of safety and effectiveness. The need for the MDR arises from various factors, including shortcomings identified in the previous regulatory framework (Medical Device Directives) and the evolving landscape of medical technologies. Rapid technological advancements in the field of medi...
Technical Documentation Requirements MDD Vs MDR
Check out the difference between MDD vs MDR MDD vs MDR MDD A MDD Technical documentation must include: MDR All Technical documentation requirements of MDD must be presented for the MDR alongside the below additional list: Device Description and Specifications Information to be supplied by the manufacturer Design and Manufacturing Information General Safety and Performance Requirements Benefit-Risk Analysis and Risk Management The benefit-risk analysis, the solutions adopted, and the results of t...
Software As a Medical Device and Its Clinical Evaluation
As technology advances across all healthcare fields, Software plays a significant role in all products. It is widely integrated into digital platforms serving both medical and non-medical purposes. Medical device software is one of three types of Software related to medical devices. The other two types of medical device software include Software that is an integral part of the medical device (medical device software) and Software used in manufacturing or maintaining the medical device. Software...
Regulation (EU) 2017/746 (IVDR): MDR IVDR Amendment Jan. 2023
Introduction Recent amendments to Regulation (EU) 2017/745, also known as the Medical Devices Regulation (MDR), have introduced significant changes to the timelines and conditions for placing certain medical devices on the market or putting them into service. This comprehensive analysis explores the key points of these amendments, focusing on the intricate timeline considerations. Paragraph 3 Amendments Paragraph 3 has undergone crucial revisions, introducing new provisions (3a to 3g) that...
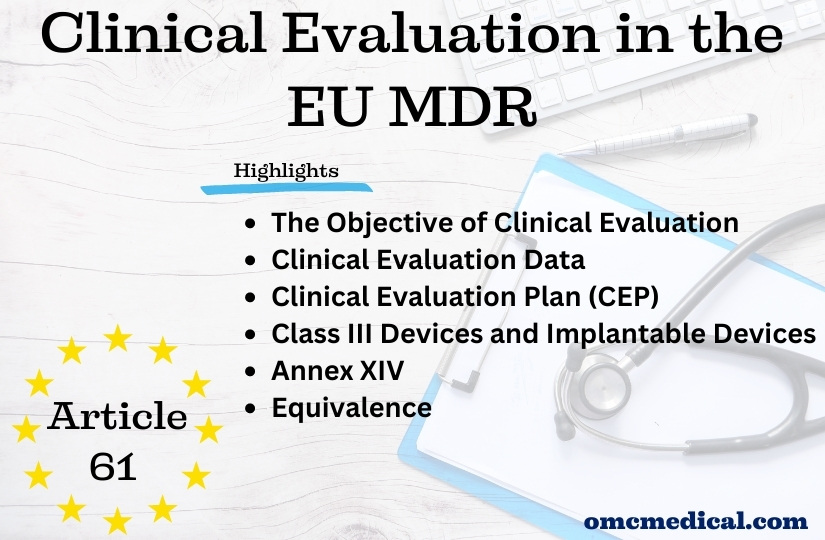
Article 61 Clinical Evaluation in the EU MDR
Article 61 Clinical Evaluation The MDR reinforces the clinical data and evaluation process (article 61 and Annex XIV), and the manufacturer must confirm the device’s conformity to fundamental health and safety requirements using reliable clinical data and evaluation. The clinical evaluation establishes the device’s safety and capacity to fulfil its intended function. It also evaluates adverse side effects and determines whether the benefit-risk ratio is acceptable. Manufacturers must...
MDCG Guidance for Manufacturers of Class I Medical Devices
A medical device’s intended use and inherent risks must be considered when determining its MDR classification. Class I devices pose less risk to patients and end users, as under the previous MDD. The new Regulation EU MDR 2017/745 has added extended rules, leading some devices to fall under Class IIa, IIb, or even III. This document is intended to guide Class I medical device manufacturers (excluding custom-made devices) that sell products bearing their name or trademark on the Union mar...
MDCG Guidance for Manufacturers of Class I Medical Devices
A medical device’s intended use and inherent risks must be considered when determining its MDR classification. Class I devices pose less risk to patients and end users, as under the previous MDD. The new Regulation EU MDR 2017/745 has added extended rules, leading some devices to fall under Class IIa, IIb, or even III. This document is intended to guide Class I medical device manufacturers (excluding custom-made devices) that sell products bearing their name or trademark on the Union mar...
Mastering Medical Device Audits: A Roadmap to Preparedness and Compliance Excellence
Facing a medical device audit can be daunting, but with meticulous preparation and strategic responses, companies can turn this challenge into an opportunity for building a robust quality system. Tips for Mastering Medical Device Audits This article provides a detailed roadmap for mastering medical device audits, covering essential steps from internal audits to adeptly handling regulatory findings. 1. Demystifying Audits Understanding the fundamental concepts behind medical device audits is cruc...