Label, Labelling vs Instructions for Use (IFU)?
1. A Label is the written, printed, or graphic information that goes on the packaging of the medical device.
2. Instructions For Use (IFUs) or Package Insert is the essential information accompanying the medical device for its safe and effective use by the user. It can be a single to multiple-page document.
3. Labelling is the content that goes on the Label or IFUs.
What are the minimum requirements for labeling?
The ISO has published many standards applicable to the medical device industry. Some of them are as below:
| Standard Number | Standard Name |
| ISO 18113 | In vitro diagnostic medical devices – Information supplied by the manufacturer (labelling) – Part 1, 2, 3, 4 and 5 |
| ISO 28219 | Packaging – Labelling and direct product marking with linear bar code and two-dimensional symbols |
| ISO 15223 | Medical Devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1 and 2 |
| ISO 3864 | Graphical symbols – Safety colours and safety signs – Part 1, 2, 3 |
| ISO 20417 | Medical devices – Information to be supplied by the manufacturer |
| ISO 14025 | Environmental labels and declarations – Type III environmental declarations – Principles and procedures |
| ISO 14021 | Environmental labels and declarations – Self-declared environmental claims (Type II environmental labelling) |
| ISO 14020 | Environmental labels and declarations – General principles |
| ISO 22742 | Packaging – Linear barcode and two-dimensional symbols for product packaging |
| There are more specific product-oriented labelling standards available. |
ISO 20417 has defines information to be disclosed by the manufacturer. Every medical device manufacturer, distributor, importer, or Authorized Representative is bound to comply with the standard before placing the device on market. The requirements are as follows:
Information on Label
1. Manufacturer details – Trade Name, address, country
2. Product description
3. Product identification – model or catalogue number, Lot number, serial number, expiry date, UDI,
4. Storage instructions
5. Operating instructions
6. Warning or precautions
7. Presence of any harmful substances (>0.1% w/w), biological origin substances, medicinal substances, nanotechnology materials
8. Electronic IFUs (if available)
9. Mention of: Single-use/ Single patient multiple-use / Reuse / Limitation on reuse
10. If Sterile and method of sterilization
11. Explanation of safety-related colors
Information on Packaging
1. Name and address of the manufacturer or an authorized representative
2. UDI
3. Production controls – lot number, serial number, expiry date
4. Model number, catalog number, commercial name
5. Mention of: Single-use/ Single patient multiple-use / Reuse / Limitation on reuse
6. Storage or special handling requirements
7. Any special requirements for battery-powered medical device
8. Contraindications, warnings, or precautions
Information in IFUs
1. General information (as above)
2. Intended Use of the medical device
3. Safety information
4. Performance of the medical device
5. Any residual risk associated with the use of the medical device or its accessory
6. Any known contraindications
7. Document control number of the IFU
8. Safe disposal information
9. Any specific instructions for handling or preparatory treatment
10. Any warnings, precautions, or limitations
11. If any accessories or indicators are provided along with the device, instructions on their use to be provided in the IFU.
12. Technical description
13. The harmonized ISO standard makes sure true and uniform information is conveyed to a lay/common person.
Global Labelling Requirements
Most countries have a mandatory requirement for the IFUs or Labels in their local language. To streamline this requirement, ISO 15223 standard provides a list of signs and symbols that depict common terms such as Manufacturer, Lot number, storage conditions, Expiry, eIFU and many more.
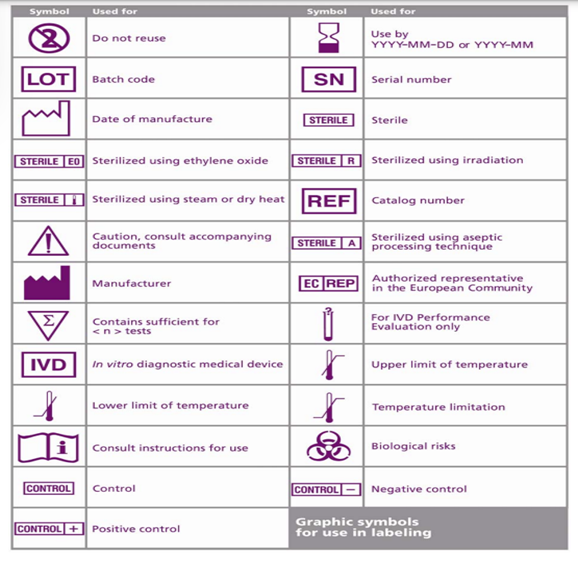
The uniform symbols help in identifying the necessary information without the language barrier. Another advantage is it saves significant label space.
Frequently Asked Questions
1. Is it necessary to follow the ISO standards?
It is advisable to develop a medical device in compliance with the applicable harmonized standards. This shall favor in smooth marketing of the product along with its competitors.
2. Is it necessary to brief the symbols in IFU when symbols from standards are used?
Yes, it is required to brief every symbol in the IFU that is used on the label of the product.
3. Can a distributor or an importer label be affixed separately apart from the main label?
Yes, it is also allowed to affix these labels separately on the product. This is because one manufacturer may have several distributors or importers within EEA.
4. Is it necessary to create dedicated labels for accessories of medical devices?
Yes, it is. Not every time the accessory is shipped along with the medical device and it is required to identify them with appropriate labels.
5. If the manufacturer wants to provide an eIFU how to indicate this on the label?
Firstly, not all the medical devices are eligible for eIFU provision. Regulation 207/2012 states what are the categories of MDs that are eligible for eIFU.
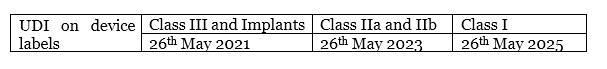
6. What is the deadline to implement UDI carrier on device labelling?
Article 123.3.f states these timelines as:
Disclaimer: Regulations/legislations are subjected to changes from time to time and the author claims no responsibility for the accuracy of information.









