If you plan to sell medical devices in India—either as a local manufacturer or a foreign company—you must register your product with the Central Drugs Standard Control Organisation (CDSCO). CDSCO is India’s national regulatory authority responsible for ensuring the safety, quality, and performance of medical devices and drugs.
In this guide, we explain the CDSCO registration process step by step, including recent updates in 2025, required documents, timelines, and key responsibilities for both Indian and foreign companies. Whether you’re new to the Indian market or renewing your compliance knowledge, this guide is designed to help you understand exactly what’s required.
Who Needs CDSCO Registration?
CDSCO registration is mandatory for:
- Indian manufacturers of notified medical devices
- Foreign manufacturers looking to import notified devices into India
If your device is listed as “notified” under the Medical Device Rules, 2017, it must go through the CDSCO registration process.
Recent CDSCO Updates (as of May 2025)
CDSCO has introduced several changes to improve efficiency and traceability:
- Neutral Code for Export: A system-generated code is now available for manufacturers exporting devices.
- Auto-Generated Compliance Certificates: Market standing and non-conviction certificates can now be generated digitally for licensed entities.
- In-Country Performance Evaluation: For new in-vitro diagnostic devices (Class B–D), clinical evaluation in India is mandatory, even if the device is approved in other countries.
These updates aim to enhance transparency and compliance monitoring, especially for higher-risk devices.
Medical Device Classification in India
Medical devices in India are classified into four risk categories, which determine the licensing path:
| Class | Risk Level | Licensing Authority |
|---|---|---|
| A | Low Risk | State Licensing Authority (SLA) |
| B | Low-Moderate Risk | SLA |
| C | Moderate-High Risk | Central Licensing Authority (CLA) |
| D | High Risk | CLA |
Correct classification is important because it affects the type of license, documentation, and review process.
CDSCO Medical Device Registration Process
CDSCO oversees the regulation and approval of medical devices in India to ensure product safety and quality. The registration steps vary depending on whether the applicant is an Indian manufacturer or a foreign entity.
Let’s get in.
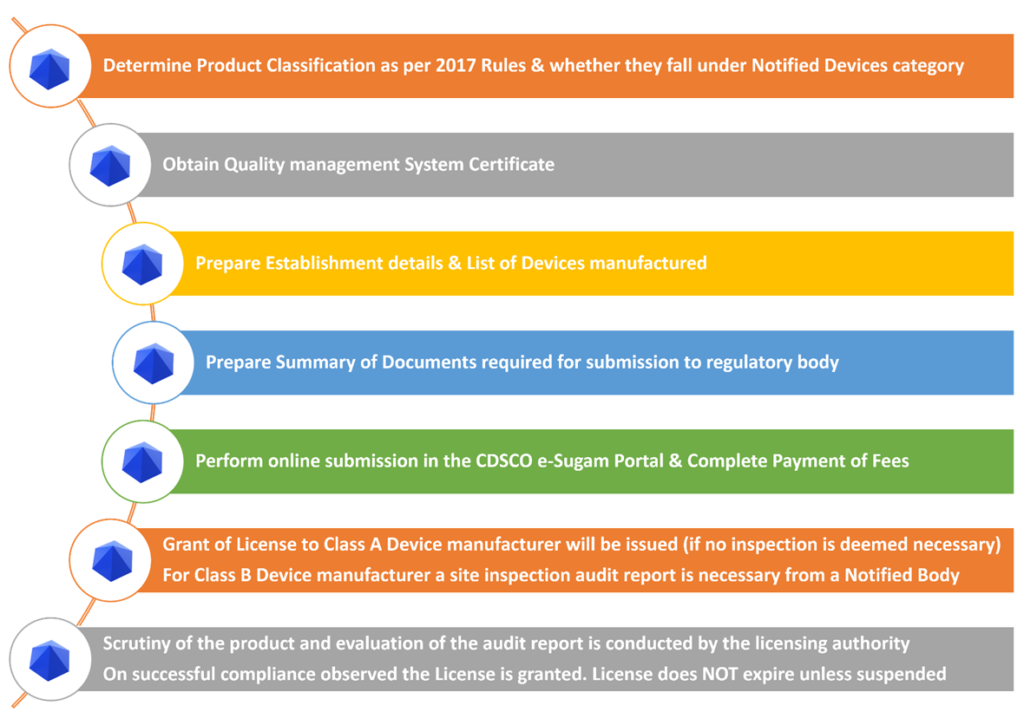
Registration Process for Indian Manufacturers
Step 1: Classify the Device
Use CDSCO’s classification list or consult an expert to identify whether your device is Class A, B, C, or D.
Step 2: Apply for Manufacturing License
Depending on classification:
- Class A & B: Apply to SLA using Form MD-3 (license issued as MD-5)
- Class C & D: Apply to CLA using Form MD-7 (license issued as MD-9)
Step 3: Comply with QMS Requirements
You must follow ISO 13485:2016 standards for quality management. Your facility may be audited by the licensing authority.
Registration Process for Foreign Manufacturers
Step 1: Appoint an Indian Authorized Agent (IAA)
The IAA must have a valid wholesale/manufacturing license in India and act as your point of contact with CDSCO.
Step 2: Submit Application through SUGAM Portal
You will need to:
- Apply for a Registration Certificate via Form MD-14 (certificate issued as Form MD-15)
- Apply for an Import License via Form MD-16 (license issued as Form MD-17)
Step 3: Prepare Technical Documentation
Your submission must include:
- Device Master File (DMF)
- Plant Master File (PMF)
- ISO 13485 certificate
- CE Certificate or Declaration of Conformity
- Free Sale Certificate (FSC)
- Power of Attorney (legalized/apostilled)
- Post-market surveillance plan
How OMC Medical Can Help
At OMC Medical, we specialize in regulatory compliance for medical devices across global markets—including India. Our team is well-versed in the latest CDSCO requirements and can support you through every step of the registration process.
We can help you with:
- ✅ Device Classification: Accurately classify your product based on CDSCO’s risk categories.
- ✅ Document Preparation: Assist in compiling the Device Master File (DMF), Plant Master File (PMF), and other required documents.
- ✅ Indian Authorized Agent Services: Act as your official representative in India, fulfilling regulatory and communication responsibilities.
- ✅ SUGAM Portal Application Support: Handle online submissions, fee payments, and follow-ups with the authorities.
- ✅ Regulatory Consulting: Advise on technical, clinical, and quality documentation including ISO 13485 compliance.
- ✅ Post-Market Compliance: Guide you on post-approval requirements such as adverse event reporting and license retention.
Whether you are a startup entering India for the first time or an established company managing multiple product registrations.
👉 Contact us today to simplify your CDSCO registration journey and ensure your medical devices reach the Indian market smoothly and compliantly.
Conclusion
The CDSCO registration process may appear complex, but following each step methodically ensures timely approvals and market access in India. Preparing a complete technical dossier, choosing the right classification, and working with an experienced Indian Authorized Agent are key to success.
Disclaimer: Regulations/legislations are subjected to changes from time to time and the author claims no responsibility for the accuracy of information.







