Regulations
ISO 20417:2021 – Information to be supplied by the Manufacturer
Engineered medical devices are introduced to the market for use in clinical scenarios. From a user’s perspective, the safe and effective usage of a medical device is highly influenced by providing appropriate accompanying information with the device labels and user...
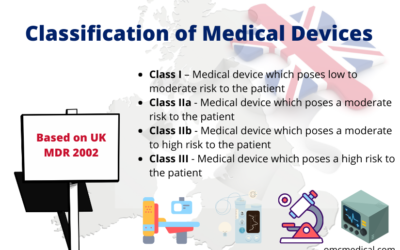
Classification of Medical Devices Based on UK MDR 2002
The classification of medical devices in the UK market will be based on UK MDR 2002 from 1st January 2021. Medical devices are classified into a particular class based on the level of risk they pose to the patient and according to the intended purpose of use. The...
In Vitro Diagnostic Regulation (IVDR) (EU) 2017/746
The IVD medical devices Regulation (EU) 2017/746 (IVDR) brings EU legislation into line with technical advances, changes in medical science, and progress in law-making. The new Regulation creates a robust, transparent, and sustainable regulatory framework recognised...
UDI DI
UDI-DI On 5 May 2017, the EU published the new EU MDR 2017/745 and IVDR 2017/746 regulations in which they formally introduced the UDI system in the EU. The UDI comprises the following components A device identifier (UDI-DI) A production identifier (UDI-PI) The Basic...
EU Requirements for Translations
Under the Medical Devices Directive (MDD), it is required that manufacturers have translations of the technical documents like Instructions for Use. However, this rule wasn't strictly followed up until MDR was brought in place. The European Union's language...
Electronic Instructions For Use Of Medical Devices
Commission Regulation (EU) No 207/2012 on electronic instructions for use of medical devices was published on 9 March 2012 and came into effect on 1 March 2013. There are 10 articles in this regulation. It establishes certain requirements for the “instructions for...
Implant Card for Switzerland
Implant Cards in Switzerland For implantable products, the manufacturer must provide the product information required under Article 16 of MedDO, the information required under Article 18 paragraph 1 EU-MDR and must include the implant card. In Switzerland, the implant...
Implant Cards for Europe
The European Union’s Medical Device Regulation (MDR 2017/745) introduces a new requirement for manufacturers producing implant cards for medical devices. As per EU MDR, implantable devices are any devices, other than the active implantable devices, that are partially...
Importance of Human Translations vs Machine Translations
Human translation Human translations produce accurate results because it involves at least one language expert. Translations, with a human touch to them, are precise because of the in-depth knowledge that translators carry Human translations are done by native...
ISO 15223-1: 2016
Many countries demand that medical devices provide textual information in their local language. When different languages are incorporated on a single label or piece of paperwork, this can cause issues with translation, design, and logistics. Users of medical devices...