EU
Commission Regulation (EU) No 207/2012 on electronic instructions for use of medical devices was published on 9 March 2012 and came into effect on 1 March 2013. There are 10 articles in this regulation. It establishes certain requirements for the “instructions for use” for the user that must be noted by the manufacturer of the device, to ensure proper and safe use of the device as well as to know about the precautions while using the device. It is meant to reduce potential risks as much...
Annex XVI – EU MDR
What is an Annex XVI product? Annex XVI products are those for which a manufacturer claims only an aesthetic or another non-medical purpose but are like medical devices in terms of functioning and risk profile. These categories of products were added in the new Regulation to establish production and surveillance standards for these previously unregulated products to protect users’ health and safety. LIST OF GROUPS OF PRODUCTS WITHOUT AN INTENDED MEDICAL PURPOSE REFERRED TO IN ARTICLE 1(2) When...
Regulatory Pathway for Clinical Investigation in the EU
Regulatory Pathway for Clinical investigation creates a set of clinical data that is crucial to understanding the effectiveness of a medical device. Clinical Investigation is done for previously CE marked devices or for research medical devices. Clinical Investigation is governed by a clinical investigation plan with an objective, methodology, and record keeping. Top 5 Steps involved in Clinical Investigation Step 1 Appointment of a Representative- Appoint a legal representative....
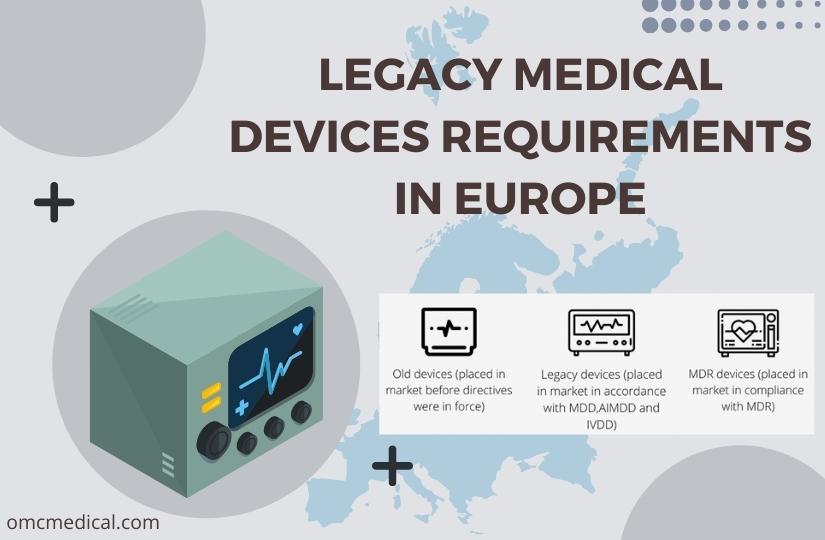
LEGACY MEDICAL DEVICES REQUIREMENTS IN EUROPE
Legacy medical devices include all previously regulated devices under the Medical Devices Directives (MDD 93/42/EEC 90/385/EEC) and In-vitro Diagnostic Devices Directive (IVDD 98/79/EC). Medical Device Coordination Group (MDCG) published the guidance documents on the Application of EU MDR to legacy devices and Legacy device management for Legacy devices. There are three terminologies to guide the manufacturers – old devices, legacy devices and MDR devices. ‘Old devices’...
ISO 20417:2021 – Information to be supplied by the Manufacturer
Engineered medical devices are introduced to the market for use in clinical scenarios. From a user’s perspective, the safe and effective usage of a medical device is highly influenced by providing appropriate accompanying information with the device labels and user instructions. An international standard that defines the information to be supplied by the manufacturer of Medical Devices is ISO 20417:2021, with the latest version as ISO 20417:2021. Besides, ISO 15223 stands for requirements that...
Quality Management System Requirements of EU MDR
Quality Management Systems (QMS) are defined by the MDR as formalised systems that document processes, responsibilities, and procedures to ensure and continually improve the standard of company activities. To obtain CE marking and market devices in European Union, an effective Quality Management System that complies with EU MDR 2017/745 is must. Article 10 of EU MDR 2017/745 explains the general obligation of manufacturers to place their products in the EU market. A...
Directive (EU) 2019/904 on the reduction of the Impact of certain Plastic Products on the Environment
Introduction Directive 2019/904 aims to mitigate the environmental and health impacts of specific plastic products, particularly in aquatic environments, while fostering the transition towards a circular economy through innovative and sustainable practices. It encompasses single-use plastic items outlined in the Annex and products made from oxo-degradable plastic and plastic-containing fishing gear. Member States are mandated to implement measures to significantly reduce the consumption of singl...
Ensuring Compliance and Quality: The Role of CE Marking in Safeguarding Patient Safety
The CE marking signifies a medical device’s adherence to stringent European Union (EU) safety regulations. This compliance process, known as CE marking compliance for patient safety, is critical in ensuring the quality and effectiveness of medical devices. By mandating rigorous assessments, it directly impacts patient safety. Manufacturers must demonstrate their devices meet essential safety and performance requirements, minimizing risks associated with malfunction, biocompatibility issues...
Staying Vigilant: Best Practices for Post-Market Surveillance under EU MDR
As medical device manufacturers navigate the landscape of post-market surveillance (PMS) under the European Medical Device Regulation (EU MDR), maintaining vigilance and adherence to best practices is crucial. Effective PMS not only ensures ongoing product safety and efficacy but also facilitates compliance with regulatory requirements. Top 7 Key Strategies for optimizing Post-Market Surveillance Here, we outline key strategies for optimizing post-market surveillance in alignment with EU MDR sta...
Safety Reporting on Clinical investigation
Under EU MDR 2017/245 Article 73, the sponsor shall report to all member states about the clinical investigations undertaken by the medical devices through an electronic system in case of any serious adverse events, device deficiency which leads to adverse events or any novel findings with regards to the event. The submission period may vary depending on the severity of the events. An initial incomplete report followed by the complete report shall be provided. The reporting procedures are...