The classification of medical devices in the UK market will be based on UK MDR 2002 from 1st January 2021. Medical devices are classified into a particular class based on the level of risk they pose to the patient and according to the intended purpose of use.
The medical devices are classified based on the classification rules in Annex IX of Directive 93/42 into one of the following four classes:
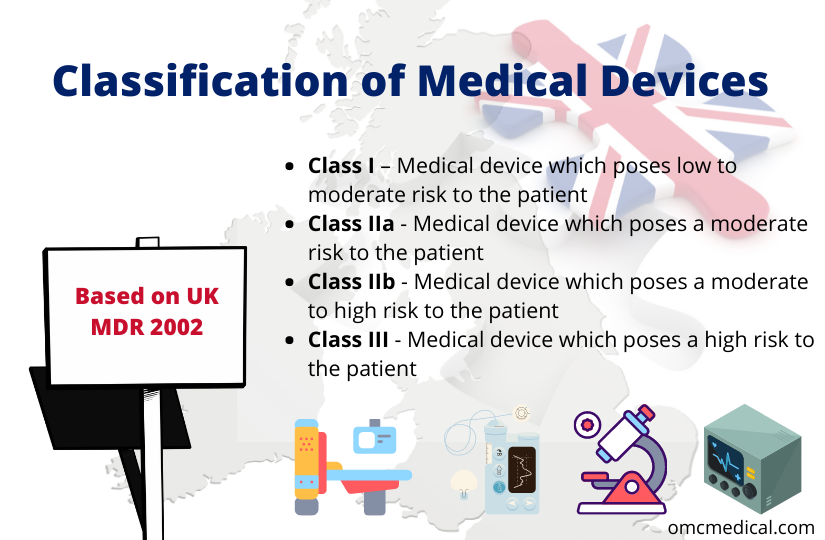
- Class I – Medical device which poses low to moderate risk to the patient
- Class IIa – Medical device which poses a moderate risk to the patient
- Class IIb – Medical device which poses a moderate to high risk to the patient
- Class III – Medical device which poses a high risk to the patient
The medical devices are classified into the above-given classes based on 18 rules mentioned in Annex IX of Directive 93/42.
These 18 rules are majorly divided into four parts which are non-invasive devices, invasive devices, active devices and special rules.
- Devices that do not penetrate inside the body fall under non-invasive
- Devices that penetrate inside the human body either through a body orifice or through the surface of the body are Invasive devices (this also includes surgically invasive devices)
- Devices that require an external energy source come under active devices
- All other devices falling outside of these categories come under Special rules
In the event of a dispute between a manufacturer and a notified body over the classification of a device, the matter shall be referred to the Secretary of State, who shall determine the classification of the device in accordance with the classification criteria set out in Annex IX of Directive 93/42.
Few Common examples of the various classes discussed above:
- Class I – Bandage, Spectacle Frames, Surgical Masks, Wheelchair
- Class IIa – Suture Needles, Hearing Aids, Surgical clamps
- Class IIb – Infusion pumps, Surgical Laser, Ventilator
- Class III – Pacemaker, defibrillator, Cochlear implants
Rules to Remember for UK MDR 2002
- Medical devices which are used in a particular combination are also individually classified into their specific class
- Software that is used to aid the operation of a particular medical device also falls into the same class as the medical device
- The medical device that is not explicitly designed for a particular part of the body is be classified based on the most critical use of that device
- If multiple rules apply to a particular medical device, the rule which involves the highest risk must be considered for the classification purpose
4 General Procedure of Medical Devices Based on UK MDR 2002
- Decide the type of device that you are about to classify into a non-invasive, invasive, active, or special category.
- Look at each UK MDR 2002 classification rule and determine which rule applies to your medical device.
- If multiple rules apply for your device apply the highest risk rule.
- If the product is combined determine its principal mode of action with respect to its intended purpose and apply the most suitable rule.
- Once the medical device is successfully classified into a particular class, the registration process or the conformity assessment for that medical device will be based on the derived risk class of the device. Hence Classification of the medical device plays an integral role in the registration process of a medical device
FAQs
For an integrated medical device that contains both hardware and software, with software being part of the entire functionality of the device, what is the basis of classification?
For an integrated medical device, the classification of the device is based on the risk imposed by the medical device in terms of safety. Classification of the software is A, B or C based on which the approach of software life cycle processes is carried out.
However, as an integrated medical device, it is classification is I, II, III & IV depending on the risk impact to the patient.
What if my medical device is a Drug-Device Combination or contains a Medicinal Product?
Where a device is intended to administer a medicinal product within the meaning of Article 1 of Directive 2001/83/EC, that device shall be governed by this Directive, without prejudice to the provisions of Directive 2001/83/EC with regard to the medicinal product.
If, however, such a device is placed on the market in such a way that the device and the medicinal product form a single integral product which is intended exclusively for use in the given combination and which is not reusable, that single product shall be governed by Directive 2001/83/EC.
The relevant essential requirements of Annex I to this Directive (93/42) shall apply as far as safety and performance-related device features are concerned.
What are the new devices that are to be classified other than the medical device?
- Accessories for medical devices can be separately classified in their own rights of use, independent of the classification applied to the main medical device.
- Products that are used in combination with other product(s), the classification rule applies separately to each individual product.
- Software, which drives a device or influences the use of a device, falls automatically in the same class.
Will the Quality Management System requirements of EU MDR be in effect for the UK in future?
At this moment, the UK MDR 2002 has adopted the EU Directive 93/42. Nevertheless, manufacturers are expected to stage up their QMS processes for reviewing their pre and post-market regulatory procedures, clinical evidence, and risk management.
Performing a gap assessment is significant to understand and prepare the road map to accelerate towards compliance for the regulations.