Cubicdesignz
Human translation Human translations produce accurate results because it involves at least one language expert. Translations, with a human touch to them, are precise because of the in-depth knowledge that translators carry Human translations are done by native speakers of a particular language or people certified in language translations . Therefore, it is culturally diverse, nuanced and is of high quality. However, this high-quality product is expensive. Machine translation Machine transla...
Implant Cards for Europe
The European Union’s Medical Device Regulation (MDR 2017/745) introduces a new requirement for manufacturers producing implant cards for medical devices. As per EU MDR, implantable devices are any devices, other than the active implantable devices, that are partially or completely implanted into the human body or are used to replace an epithelial surface or the surface of the eye, by clinical intervention, and are meant to stay in place after the procedure. The device that is intended to be pa...
EU Classification of Medical Devices with Examples
EU Classification of Medical Devices Around the world, the definition of medical devices varies. Still, generally, a medical device is any instrument, equipment, machine, appliance, implant, reagent for in vitro use, software, material, or other similar or related product intended by the manufacturer to be used either alone or in combination for various medical purposes. Medical devices range from low-risk products such as thermometers to high-risk devices such as pacemakers. In the European Uni...
Top 7 Guidance On Class I Medical Devices
The EU MDR 2017/745 imposes more stringent requirements for Class I devices. Under the new regulations, Manufacturers must righty classify a medical device and provide technical documentation following the device class. The risk class under MDD could change under MDR. In some cases, medical devices could be up classified from Class I medical devices to Class II a/b medical devices or Class III medical devices. Please read our article on the classification of medical devices to understa...
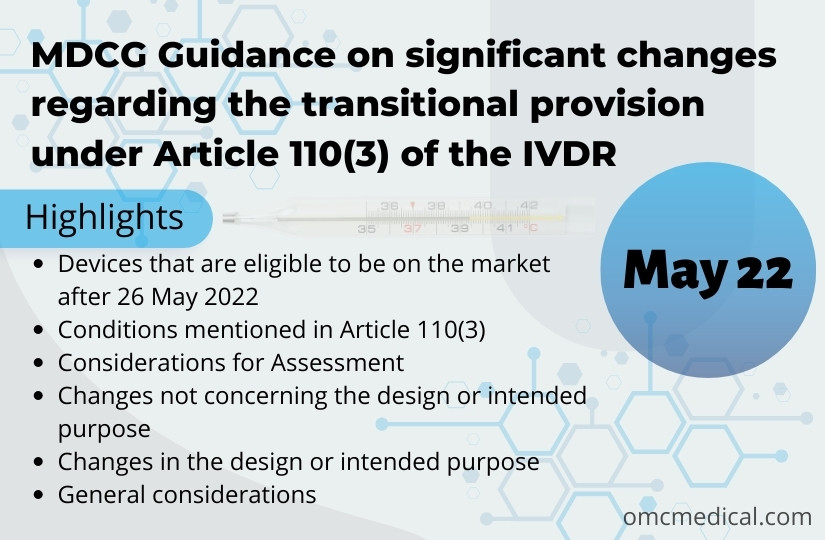
MDCG Guidance on Article 110(3) of the IVDR
MDGC Guidance on significant changes regarding the transitional provision under Article 110(3) of the IVDR aims to provide clarification on the concept of ‘significant changes in the design and intended purpose’. This article applies to manufacturers of devices that comply with Directive 98/79/EC and is placed on the market or put into operation after the transition period ends on May 26, 2022, irrespective of the involvement of notified body under the IVDD. It is important to note that IVDR...
Corrective And Preventive Action (CAPA)
“Corrective action” is the immediate address of an issue, whereas “Preventive action” is addressed to reduce or stop the recurrence of the same error from happening again. What is a CAPA? Corrective And Preventive Action consists of a system of procedures to help improvise the non-conformances, undesirable outcomes and even field safety corrective actions of an organisation. Corrective And Preventive Action must include the following steps: CAPA is a time-bound activity. Corrective And P...
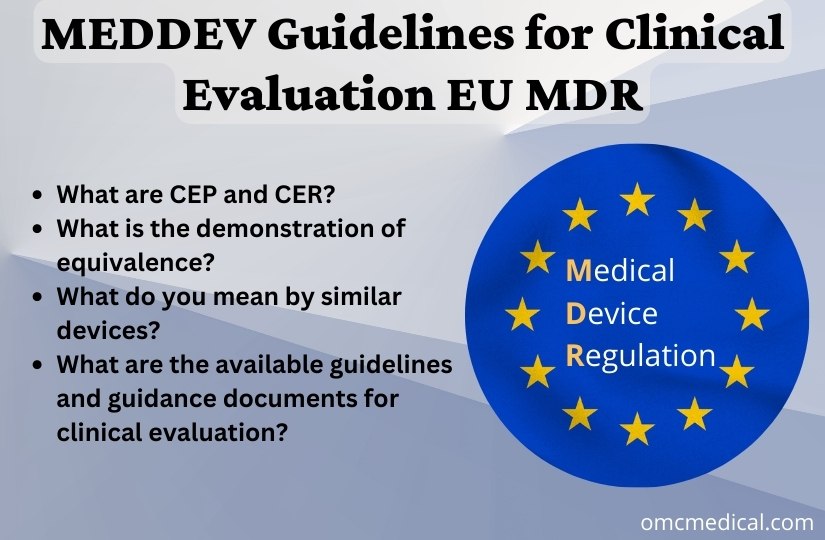
MEDDEV Guidelines for Clinical Evaluation EU MDR
During the conformity assessment, the manufacturer must submit the complete information material (labelling, IFU (Instructions for Use), any promotional materials and other relevant documents), Clinical evaluation plan & report with the available clinical data, which includes (General Safety and Performance Requirements) GSPR, intended target groups and purpose, qualitative and quantitative aspects of clinical safety, performance procedures and other risk factors involved. The manufacturer m...
IMDRF guide on Post Market Clinical Follow-up Studies (PMCF)
PMCF is a continuous process that updates the manufacturers’ clinical evaluation during the Post-market surveillance. They must collect user feedback, clinical data, and all other clinical experiences. This article provides an overview of the design, implementation, and appropriate use of PMCF studies. these are an integral part of PMS reports. There may be some data limitations during the premarket phase, like the duration of the investigation and the number of subjects involved or restri...
EU Notified Body
A notified body is an independent organisation designated by an EU country to assess the conformity of products before being placed on the market. Under EU MDR 2017/745, the notified body is also called a conformity assessment body. A conformity assessment body (CAB) or a notified body is responsible for carrying out the conformity assessment procedure mentioned in the applicable Regulation. What does a notified body do? A notified body assesses whether the product conforms to the require...
The combination of medical devices and medicinal products based on MDCG 2022-5
This article focuses on the distinctions between the MDD and MDR, considering the terminologies and real-world examples of substance-based devices and combinations of medical and medicinal products. MEDDEV (Medical Devices) 2.1/3 Rev3 guideline for “Borderline products, drug-delivery products, and medical devices incorporating, by an integral part, an ancillary medicinal substance or with ancillary human blood derivative” got replaced with MDCG 2022-5 ” Guidance on the borderli...