Cubicdesignz
The FDA 510(K) Pre-market notification submission as per 21 CFR 807 Subpart E is to be adopted by manufacturers to receive FDA clearance to market medical devices or for commercial distribution in the U.S. This review is done by FDA’s Centre for Devices and Radiological Health (CDRH). A 510(K) submission allows medical devices to be “FDA Cleared” and not FDA Approved. The route to 510(K) must be carefully investigated by the manufacturer through a step-by-step process which allows determin...
De Novo Request | FDA
The De novo request is a simpler marketing pathway to classify novel medical devices that provide a reasonable assurance of safety and effectiveness for the intended use and do not already have a predicate device on the market. FDA also declares that the devices marked as Class I or II as per De novo request can be further used as a predicate device for future premarket 510(k) notifications. De Novo Request Procedure There are two ways to submit a De Novo request to the FDA for a risk-based eval...
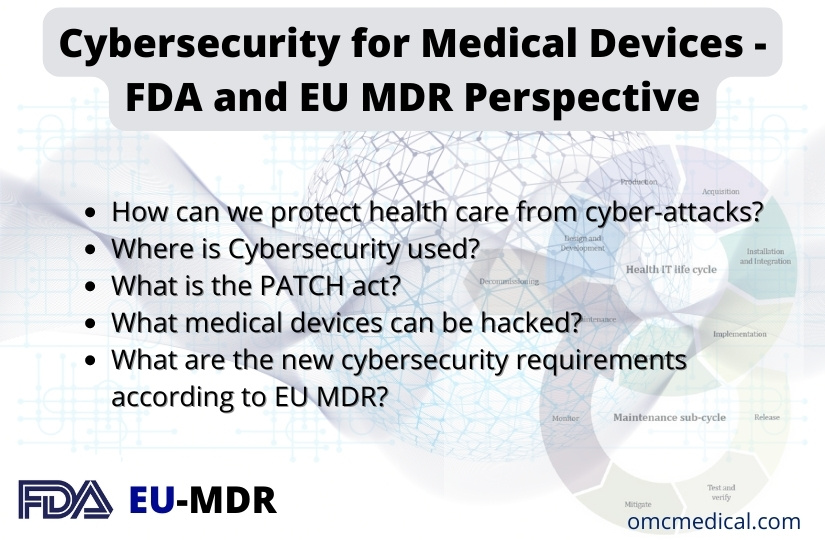
Cybersecurity for Medical Devices – FDA and EU MDR Perspective
Cybersecurity for Medical Devices FDA –Food and Drug Administration The revolution in the digital sector has resulted in the Internet of Things (IoT), Software as a Medical Device (SaMD), Internet of Medical Things (IoMT) and other connected devices permeating the healthcare environment, both in hospital and home, has ended up with the possibility of cyberattacks and intrusions against the connected medical devices and the networks to which such a device is connected. Most Medical devices are...
FDA Post-Market Surveillance (PMS) for Medical Devices
FDA Post-Market Surveillance (PMS) is a regulatory requirement under 21 CFR Part 822, designed to ensure the long-term safety and effectiveness of medical devices after they have been released into the market. It helps manufacturers identify potential risks, track real-world device performance, and take necessary corrective actions to maintain compliance with FDA PMS regulations. What is FDA Post-Market Surveillance (PMS) for Medical Devices? FDA PMS refers to the systematic monitoring of medica...
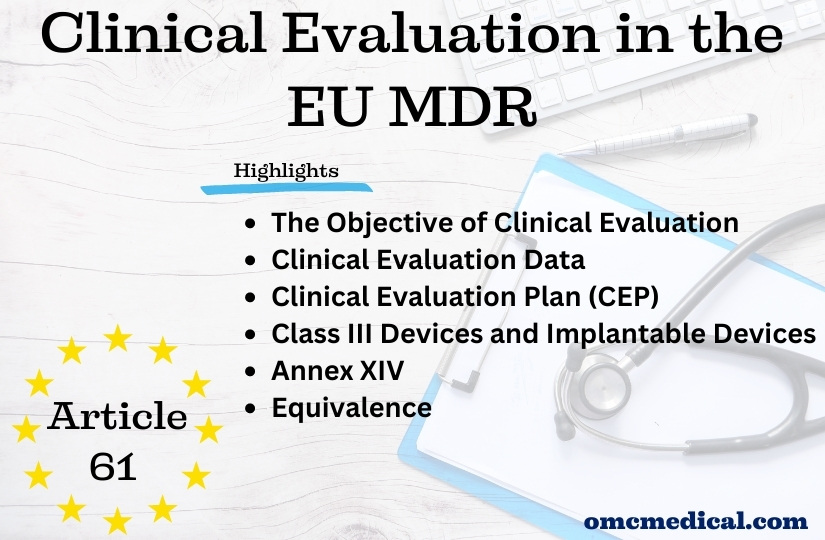
Article 61 Clinical Evaluation in the EU MDR
Article 61 Clinical Evaluation The MDR reinforces the clinical data and evaluation process (article 61 and Annex XIV), and the manufacturer must confirm the device’s conformity to fundamental health and safety requirements using reliable clinical data and evaluation. The clinical evaluation establishes the device’s safety and capacity to fulfil its intended function. It also evaluates adverse side effects and determines whether the benefit-risk ratio is acceptable. Manufacturers must...
Mastering Medical Device Audits: A Roadmap to Preparedness and Compliance Excellence
Facing a medical device audit can be daunting, but with meticulous preparation and strategic responses, companies can turn this challenge into an opportunity for building a robust quality system. Tips for Mastering Medical Device Audits This article provides a detailed roadmap for mastering medical device audits, covering essential steps from internal audits to adeptly handling regulatory findings. 1. Demystifying Audits Understanding the fundamental concepts behind medical device audits is cruc...
Navigating Medical Device Regulations: A Comprehensive Guide to Market Access in Europe, Switzerland, and the UK
Introduction and Guide to Market Access in Europe, Switzerland, and the UK In the intricate landscape of medical device regulations, successfully bringing products to market requires a thorough understanding of the varying requirements in different regions. This market access guide provides comprehensive insights into the European Union (EU), Switzerland, and the United Kingdom (UK) regulations, shedding light on crucial aspects such as Authorized Representatives, Importers, and other economic o...
Regulation (EU) 2017/746 (IVDR): MDR IVDR Amendment Jan. 2023
Introduction Recent amendments to Regulation (EU) 2017/745, also known as the Medical Devices Regulation (MDR), have introduced significant changes to the timelines and conditions for placing certain medical devices on the market or putting them into service. This comprehensive analysis explores the key points of these amendments, focusing on the intricate timeline considerations. Paragraph 3 Amendments Paragraph 3 has undergone crucial revisions, introducing new provisions (3a to 3g) that...
EU MDR Unveiled: What You Need to Know About Medical Device Regulation in Europe
Introduction about Medical Device Regulation in Europe Introducing the Medical Device Regulation in Europe addresses several challenges. It aims to enhance medical device regulation in Europe to ensure a higher level of safety and effectiveness. The need for the MDR arises from various factors, including shortcomings identified in the previous regulatory framework (Medical Device Directives) and the evolving landscape of medical technologies. Rapid technological advancements in the field of medi...
The Role of Notified Bodies in Assessing Clinical Evaluation Reports (CERs) under EU MDR
Clinical evaluation is a continuous procedure that happens at every stage of a medical device’s life cycle. It is typically carried out initially in the course of developing a medical device to determine what data must be produced to gain access to the market. The first CE-marking must include a clinical evaluation, and it must be regularly updated going forward. Clinical evaluation is essential and significant because it guarantees that assessments of the device’s performance and sa...