The amended ‘Blue Guide’ on the application of the product rules 2022’ (“Blue Guide”) was released by the European Commission on June 29, 2022.
The Blue Guide allows a better understanding of EU product regulations and their uniform and coherent application across various sectors throughout the EU single market.
The Blue Guide has undergone significant changes, including the definition of new terms, the addition of information on which economic actors will be responsible for compliance in a complicated product supply chain, and the incorporation of Regulation (EU) 2019/1020 on market surveillance and product compliance.
Technical Documentation of The ‘Blue Guide’
The manufacturer must compile the technical documentation, including details proving the product complies with all relevant specifications.
If the law mandates a conformity assessment process based on a quality system, this paperwork may be a component of the quality system documentation.
Regardless of the product’s origin or location, technical documentation must be available when the product is put on the market.
The technical documentation must be preserved for ten years following the date the product was placed on the market. The manufacturer or the authorised representative based in the Union oversees this.
The documentation must include
- Description of the product
- Intended use of the product
- Design and manufacture of the product
- Operation of the product
The requirements in Annex II of Decision No. 768/2008/EC concern the technical documentation necessary to demonstrate the product’s compliance with the relevant harmonisation legislation.
If only part of the harmonised standard is applied or does not cover all relevant essential requirements, then the way applicable essential requirements not covered by it are dealt with should be documented in the technical documentation.
The technical documentation must reflect all versions of the product, including the changes made, information on how the various conformity assessments can be identified, and information on how the different versions of the product can be identified to avoid scenarios in which, during a product’s life, a market surveillance authority must deal with product versions for which the technical documentation given to it does not apply.
Even if it isn’t explicitly stated in the Union harmonisation legislation, the documentation must always be in a language the notified body can understand.
EU Declaration of Conformity
As part of the conformity assessment process outlined in the Union harmonisation legislation, the manufacturer or the authorised representative formed within the Union must prepare and sign an EU Declaration of Conformity.
This document is required to show the product’s compliance with the applicable legislation requirements.
Unless the legislation specifies otherwise, the manufacturer must maintain the EU Declaration of Conformity for 10 years after the product is placed on the market. The importer is accountable for the Declaration of Conformity for products they have brought in.
It is necessary to keep the EU Declaration of Conformity updated. Even if they are produced in series, each product has its own EU Declaration of Conformity.
The version of the EU declaration of conformity must be updated for products put on the market after any modifications have been made to any elements of the EU declaration of conformity.
Either the model declaration found in Annex III of Decision No. 768/2008/EC or a model declaration directly annexed to the in question sectoral Union harmonisation legislation have to be referred to understand the contents in the EU Declaration of Conformity.
The declaration must include enough details to allow the identification of all the products it covers, whether in the form of a document, label, or equivalent.

To ease the administrative burden on economic operators, where multiple pieces of Union harmonisation legislation apply to a product, the manufacturer or the authorised agent must produce a single declaration of conformity.
The surveillance authority must access the EU declaration of conformity upon request. The declaration must always be made in the language(s) that the Member state(s) where the product is marketed requires.
Marking requirements
Before many products may be marketed on the European market, a CE Mark must be affixed to them. The label identifies a product as:
- Complies with the relevant standards of European product directives
- Satisfies all requirements outlined in Europe’s applicable, recognised, and harmonised performance and safety standards.
- Appropriate for its intended use and won’t threaten people or property
The CE Mark is mandated conformity marking used by the European Union (EU) to control the sale of goods inside the European Economic Area (EEA).
A manufacturer certifies that their products conform with the EU’s New Approach Directives by placing the CE mark on them. These directives include products made in or intended for sale in the EEA and those sold in the EU. As a result, the CE symbol is identifiable everywhere, even by those unfamiliar with the EEA.
The manufacturer is ultimately in charge of the product’s compliance with the provisions of the Union harmonisation legislation and the use of the CE marking, regardless of whether they are based inside or outside the Union.
The manufacturer has the right to direct an authorised agent to apply the CE marking on his behalf. By placing the CE marking on a product, a manufacturer certifies that it complies with all applicable regulatory requirements for CE marking, on his sole responsibility.
Suppose the importer or distributor or another operator places products on the market under his name or trademark or modifies them. In that case, he then takes over the manufacturer’s responsibilities, including the responsibility of affixing the CE marking.
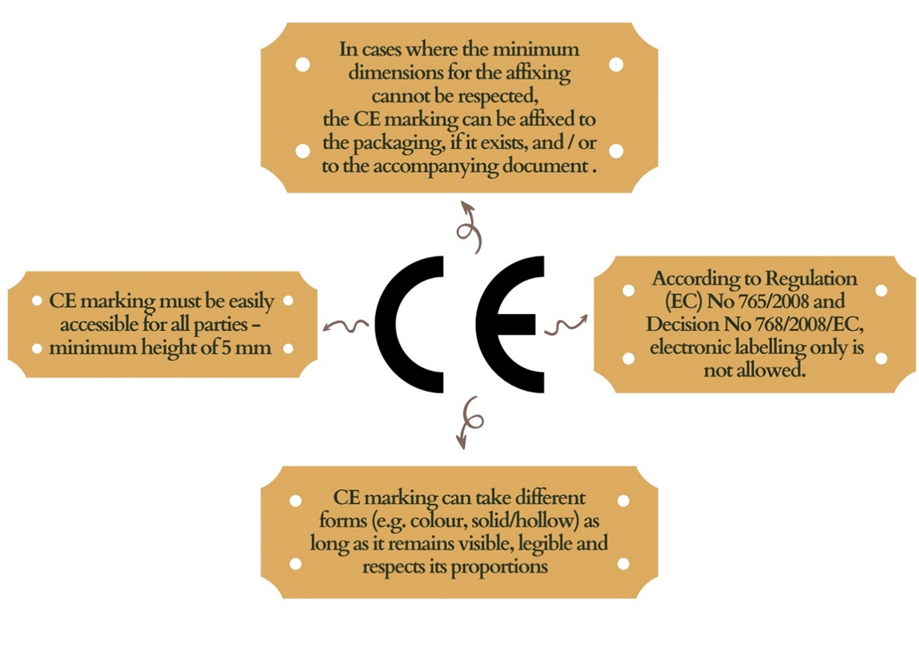
The definition, the format, and the general guidelines governing the CE marking are outlined in Regulation (EC) No. 765/2008. Procedures for conducting conformity assessments that result in its affixing are outlined in Decision No. 768/2008/EC.
The Regulation (EC) No 765/2008 and Decision No 768/2008/EC’s guiding principles are primarily upheld by the sectoral Union harmonisation legislation requiring the application of the CE marking.
If a notified body participates in the production control phase following the appropriate Union harmonisation law, its identification number must come after the CE marking.
If the legislation so demands, the manufacturer or the authorised agent must attach the identifying number under the supervision of the notified authority.
A notified body may participate in the production stage depending on the conformity evaluation techniques. Only if it engages in manufacturing must the notified body’s identification number come after the CE marking.
| CE marking appears on products without an identification number | CE marking appears on products with an identification number |
| Either no notified body intervened in the design or production phase (module A) | Either upon manufacturer’s choice, a notified body intervened in the production phase (modules A1, A2) |
| Upon manufacturer’s choice, the in-house accredited body intervened in the production phase (modules A1, A2) | A notified body intervened in the design phase (module B), and upon the manufacturer’s choice, a notified body (not necessarily the same one but the one whose identification number appears) intervened in the production phase (modules C1, C2 following module B) |
| A notified body intervened in the design phase (module B), but no notified body intervened in the production phase (module C following module B); | A notified body intervened in the design phase (module B), and a notified body (not necessarily the same one but the one whose identification number appears) intervened in the production phase (modules C1, C2, D, E, F following module B) |
| A notified body intervened in the design phase (module B), and upon the manufacturer’s choice, the in-house accredited body intervened in the production phase (modules C1, C2 following module B) | A notified body intervened in the design and production phase (modules D1, E1, F1, G1 H, H1) |
Modules for Conformity Assessment
A conformity assessment is any procedure by the manufacturer to evaluate a product, system, service, or perhaps even a person’s compliance with the standards and specifications outlined in a standard or specification.
Testing or inspection is frequently used for verification. Conformance assessments are performed on products during the design and manufacturing phases.
A conformity assessment procedure’s primary goal is to show that products that have been put on the market adhere to the standards set out in the existing legislation.
Conformity assessment processes comprise one or two conformity assessment modules under Union harmonisation legislation. A conformity assessment encompasses both the design and production phases since products are subject to conformity evaluation during both phases.
In contrast, a module may cover just one of the two phases or both. A “horizontal menu” of conformity assessment modules and how processes are constructed from modules is outlined in Decision No. 768/2008/EC.
Union harmonisation legislation creates conformity assessment processes either by foreclosing on the manufacturer’s options or by defining a range of options from which the manufacturer must select.
The manufacturer is responsible for conformity evaluation. However, a third party must be included in the compliance evaluation process if required by the applicable legislation.

FAQs
What is the significance of CE marking?
By applying the CE marking to a product, the manufacturer declares solely on his responsibility that the product complies with the essential requirements of the applicable Union harmonisation legislation requiring its application and that the relevant conformity assessment procedures have been completed. Products bearing the CE mark are presumed to comply with the applicable Union harmonisation legislation and thus have free circulation in the European Union.
Can I, as a manufacturer, personally affix the CE marking to my products?
After the required conformity assessment procedure has been completed, the manufacturer or his authorised representative can apply the CE marking. This means that the product must go through the conformity assessment procedure outlined in one or more of the relevant Union harmonisation acts before being given the CE marking and put on the market. The latter determines whether the manufacturer himself may conduct the conformity assessment or whether the involvement of a third party (the notified body) is necessary. The published ‘Blue guide’ helps product manufacturers understand how to place their products in conformity with the applicable product regulation.
Disclaimer: Regulations/legislations are subjected to changes from time to time and the author claims no responsibility for the accuracy of information.
