South Korea Cosmetic Product Registration

COSMETIC REGISTRATION IN SOUTH KOREA
Cosmetics fall under two categories: functional cosmetics and regular cosmetics. MFDS reviews only functional cosmetics for pre-market approval. For all other regular cosmetics, the MFDS authorized the Korea Pharmaceutical Traders Association (KPTA) to review and certify import permission requests submitted by the Korean importer.
Regulatory AuthorityMinistry of Food and Drug Safety (MFDS)
https://www.mfds.go.kr/eng/index.do
Local RegulationCosmetics Act and its Enforcement Decree
Regarding functional cosmetics, any Responsible Seller planning to manufacture or sell such products through manufacturing or importing must undergo evaluation by the Minister of the Ministry of Food and Drug Safety (MFDS). Alternatively, they must submit a safety and effectiveness report for each product to the Minister of the MFDS, as outlined in Article 4 of the Cosmetics Act (Examination, etc. of Functional Cosmetics). This requirement also extends to any revisions made to previously evaluated aspects.
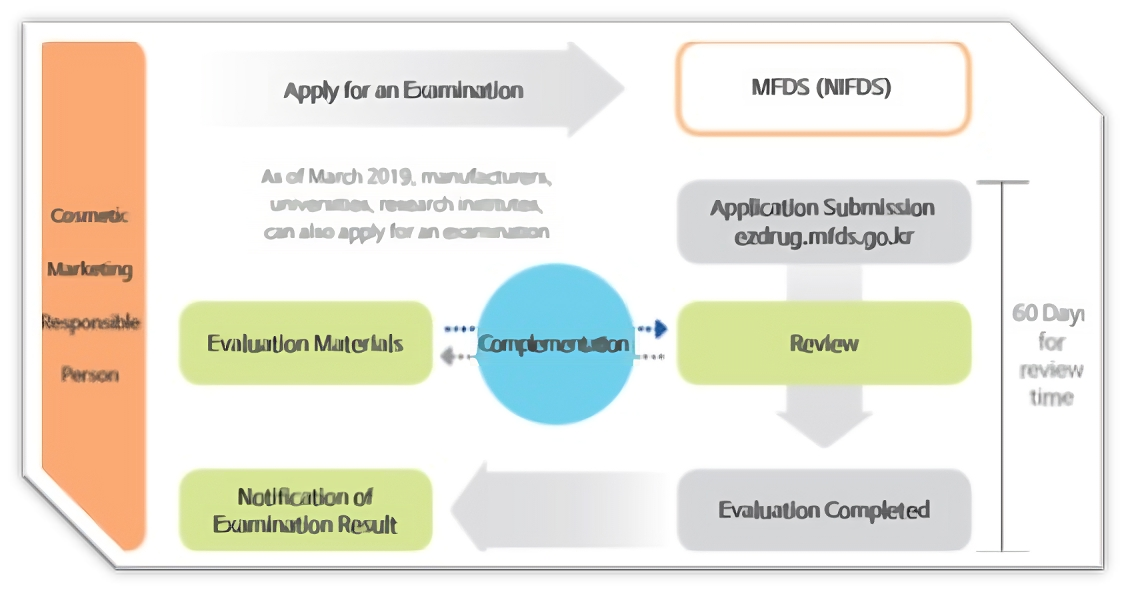
Certain ingredients have already received approval from the MFDS for use in specific types of functional cosmetics, albeit with restrictions. In such cases, if a manufacturer or importer intends to market a functional cosmetic containing only these approved ingredients, they are not required to provide clinical or efficacy data. The approval for these products can be obtained within approximately 7 days through a submission procedure.
Conversely, if the manufacturer/importer seeks to utilize unapproved ingredients, they must submit clinical and efficacy data, along with identification of the active ingredients, when seeking approval from the MFDS. This approval process may take 4-6 months to complete.
Timeframe and FeesJoin hundreds of companies who trust OMC Medical for their regulatory needs.