In recent years, the beauty and cosmetics industry has witnessed exponential growth worldwide, breaking down geographical barriers and creating a global marketplace.
One country that has embraced this transformative trend is Saudi Arabia, where the beauty industry has flourished, reflecting the changing cultural landscape. However, with growth comes the need for regulations to ensure consumer safety and product quality.
Legal Framework for Saudi Arabia Cosmetic Regulations
Saudi Arabia has established a robust legal framework to govern the cosmetics industry, ensuring that products meet stringent safety, efficacy, and quality standards. The Saudi Food and Drug Authority (SFDA) plays a pivotal role in regulating cosmetics and ensuring manufacturers follow the Cosmetic Regulations in Saudi Arabia.
The SFDA operates in accordance with international best practices, harmonizing its regulations with global standards to facilitate the import and sale of cosmetics in Saudi Arabia.
The Law of Cosmetic Products promulgated by Royal Decree number M/49 on 18/6/1436 AH is the Cosmetic Regulation followed in Saudi Arabia.
Registration Process for Cosmetic Regulations in Saudi Arabia
One of the primary requirements for cosmetic products entering the Saudi market is registration with the SFDA. Manufacturers and distributors must submit detailed documentation about their products, including information on ingredients, formulations, and safety assessments.
This rigorous registration process guarantees that cosmetics comply with Saudi regulations and do not pose any health risks to consumers.
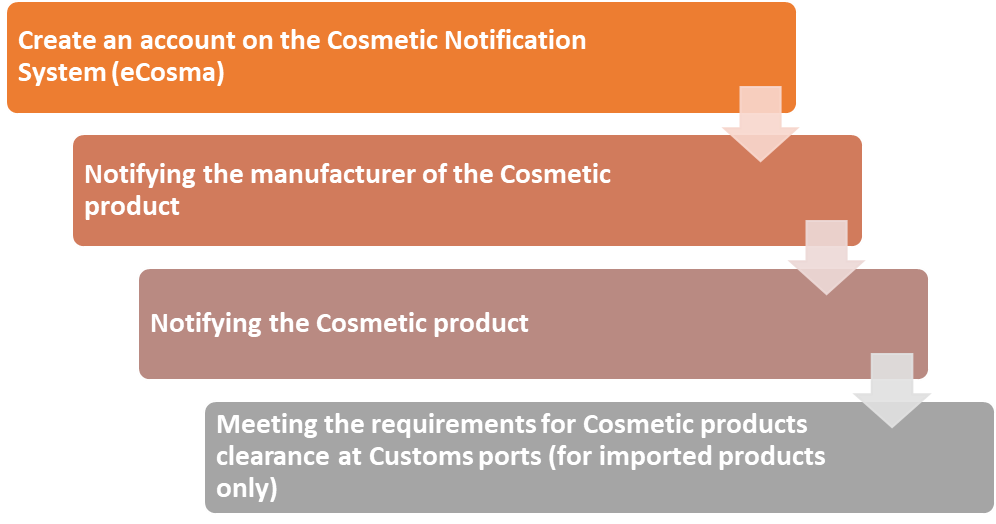
Notifying is listing cosmetic products in the SFDA records via the Cosmetic Notification system (eCosma) and does not mean SFDA has evaluated the product safety.
Notifying aims to create a comprehensive database of cosmetic products manufactured locally or imported by recording them in SFDA records, and the notifier (responsible person) shall comply with the validity of the information entered and the safety of the products.
Product Testing and Safety
Cosmetic products must undergo rigorous safety assessments to meet the SFDA’s standards. This includes testing for microbiological contamination, stability, and compliance with permissible limits of certain ingredients.
Manufacturers are responsible for providing evidence of product safety during the registration or notification process.
Halal Certification
Given the cultural and religious context of Saudi Arabia, the concept of Halal (permissible in Islam) is of utmost importance. Like other consumer goods, cosmetic products may undergo Halal certification to ensure they adhere to Islamic principles.
While Halal certification is not mandatory, many manufacturers seek it as a mark of authenticity and to appeal to the sensibilities of the Saudi consumer base.
Ingredient Restrictions
Saudi Arabia imposes strict regulations on the ingredients used in cosmetics. Certain substances are prohibited or restricted due to their potential harm to consumers. The SFDA maintains a list of restricted ingredients, and compliance is mandatory for all cosmetic products sold in the country.
This includes a comprehensive ban on ingredients that are considered hazardous or pose a risk to human health. The notifier (responsible person) must ensure the integrity of the entered data and the product’s safety.
Note that the Authority published a list of the banned products and restricted use in the cosmetic products in addition to a list of preservatives and colouring items allowed in the Cosmetic Products Website in accordance to article four of the Implementing Regulation of the Cosmetic Products Law.
Labelling Requirements for Cosmetic Regulations in Saudi Arabia
Precise and accurate labelling is crucial for consumer safety and informed choices. The SFDA mandates that all cosmetic products bear labels in Arabic, providing essential information such as product name, ingredients, instructions for use, and contact details of the manufacturer or distributor. Compliance with these labelling requirements is essential for market access and consumer trust.
- When submitting cosmetic product notifications, a clear image (one or more) of the product from all sides that shows its information and substitutions in a visible and readable way is required. The image shall include the internal and external product label and its internal brochure, if any. The images can be presented in one of the following forms: PNG, JPG, TIFF, or PDF.
- The cosmetic product label shall confirm with the Cosmetic Products Legislation and the Standard of Safety Requirements for Cosmetic Products and personal care No. GSO1943/20016.
- The product label must contain its barcode and shall be entered into the cosmetic notification system, taking into account the product’s size and colour.
Post-Market Surveillance
To ensure ongoing consumer safety, the SFDA conducts post-market surveillance to monitor the safety and quality of cosmetic products already in circulation.
This involves regular inspections, product sampling, and analysis to identify potential risks or violations. Manufacturers and distributors must cooperate fully with the SFDA during these surveillance activities to maintain compliance.
Conclusion
Saudi Arabia’s cosmetics industry thrives, driven by a dynamic consumer base seeking high-quality, safe, and culturally appropriate products. The regulatory framework established by the SFDA plays a pivotal role in maintaining the integrity of the market and ensuring that cosmetics meet the highest standards.
As the industry continues to evolve, staying abreast of the regulatory landscape is imperative for businesses seeking success in Saudi Arabia’s beauty sector.
Beauty truly knows no borders, and by understanding and adhering to the regulations, companies can contribute to the flourishing beauty industry in the Kingdom while prioritizing consumer well-being.
