The FDA 510(K) Pre-market notification submission as per 21 CFR 807 Subpart E is to be adopted by manufacturers to receive FDA clearance to market medical devices or for commercial distribution in the U.S.
This review is done by FDA’s Centre for Devices and Radiological Health (CDRH). A 510(K) submission allows medical devices to be “FDA Cleared” and not FDA Approved.
The route to 510(K) must be carefully investigated by the manufacturer through a step-by-step process which allows determining if the regulatory pathway chosen for the Medical Device’s FDA access is in the right direction.
Step 1: Decision Criteria Checklist
The assessment checklist helps the manufacturer to arrive at a decision if they are eligible or fall under the rules to submit an FDA 510(K) application.
| Criteria | 510(K) Submission required? |
| Are you a domestic manufacturer willing to commercially distribute your product in the U.S? | ü |
| Are you developing specifications for a Finished device and you have an external firm/contractor who manufactures the device based on your specifications? | ü |
| Are you a repacker or re-labeller who makes significant changes to the device operations such as changing label contents/warnings/safety signs / operating conditions to the original device label prior to sale to the market? | ü |
| Are you a foreign manufacturer? | ü |
| Are you making changes to an existing 510(K) cleared finished device where the changes could significantly affect the device’s safety and effectiveness? | ü |
| Are you making changes to the intended use of the medical device? | ü |
| Do you manufacture accessories for a medical device that are sold directly to the end-user as replaceable/serviceable parts? | ü |
| Do you sell unfinished devices or components to another firm that places the Finished device for sale using your components in their device? | û |
| Are you willing to introduce your finished devices for clinical trials only to the market? (this means you are only subjecting your device for clinical trials and not commercially distributing them) | û |
| Are you acting the role of a distributor for a domestically manufactured device by affixing only labels indicating “distributor” or “manufacturer” details? | û |
| Are you an “Importer” who is willing to import a foreign manufactured device and that device has already been 510(K) cleared? | û |
| Is your device either Class I or Class II and falls under the Medical Device Exemptions 510(K) and GMP requirements of the FDA? | û |
Step 2: Device Classification
The next step toward submission is to verify how the medical device is classified under the FDA classification regulations.
There are different generic types of devices identified by the FDA and placed under 3 categories of regulatory classes based on the risk posed by the medical device and the level of controls necessary for the safety and effectiveness of the device.
Class I Devices Low-Risk Devices – General Controls
- With Exemptions
- Without Exemptions
The above states that certain class I low-risk devices are exempted from the “General Controls”.
Class II Devices Moderately Risk Devices – General Controls and Special Controls
- With Exemptions
- Without Exemptions
The above states that certain class II devices are exempted while others fall under the provisions of “General and Special Controls”.
Class III Devices High-Risk Devices – General Controls and Pre-market Approvals
- Pre-Market Approval
Step 3: Determine if your device is Substantially Equivalent to a Predicate Device
510(K) submission is applicable only for devices that can claim Substantial Equivalence (SE) to a predicate device. Below flowchart is an illustration that helps to clearly understand the decision route:
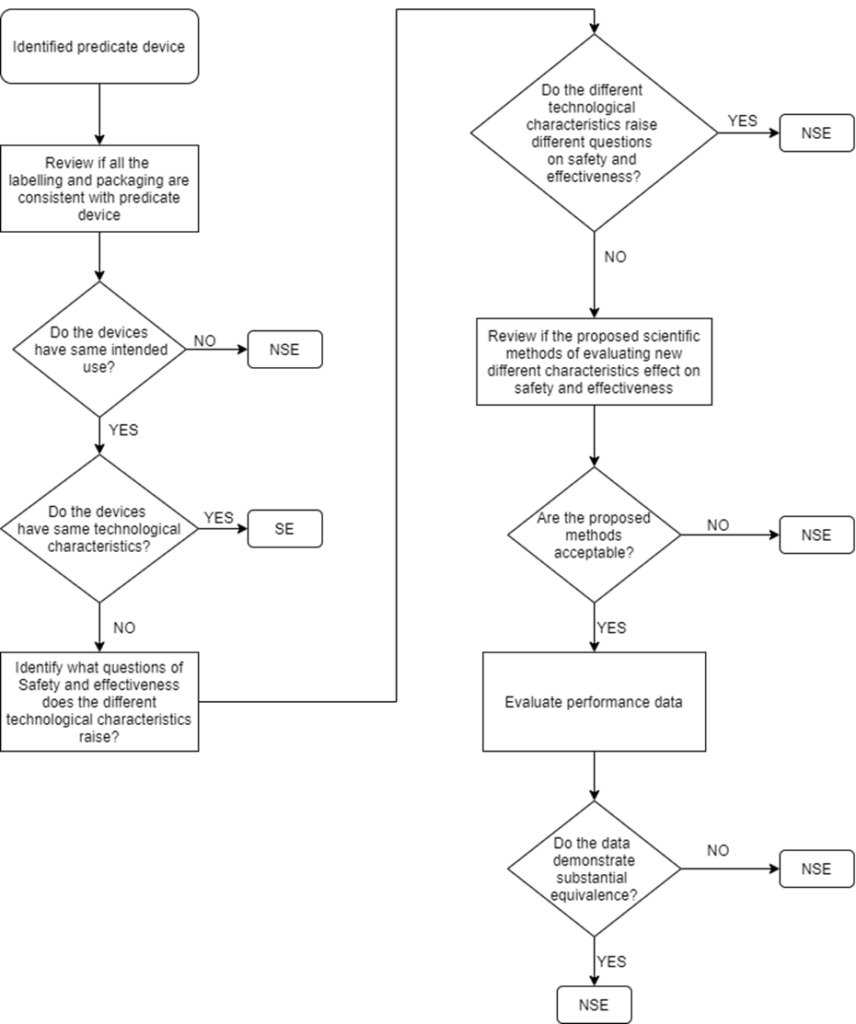
“SE” – Substantial Equivalent
“NSE” – Non-Substantial Equivalent
Multiple Predicate Devices
In certain cases, the manufacturer may identify more than one predicate device i.e., multiple predicates. In such cases, the primary predicate refers to the one that is most similar to the below factors:
- Intended Use
- Indications for use
- Technological characteristics
The manufacturer is recommended to identify the most appropriate primary predicate device with a well-supported decision document.
Supporting Documents to Claim Substantial Equivalence
The following are required by the manufacturer but not limited to, while demonstrating the most appropriate predicate device and that the new device to be submitted for 510(K) is a substantial equivalent to a predicate device.
- Intended Use
- Indications for Use
- Technological Characteristics (similarities, differences and whether the differences pose different questions on safety and effectiveness)
- Performance data to support substantial equivalence (biocompatibility testing, Electrical safety and Electromagnetic Compatibility, Software verification and validation testing, mechanical testing, clinical study, animal study, if applicable)
- A declaration of conformity to recognised standards applicable to the medical device
Refer to the Section 807.92 content and format of a 510(K) summary.
Step 4: Determine the Type of 510(K) Submission
Within the 510(K) applications, there are 3 categories of submissions as discussed below
| SL. | Submissions | Type of 510(K) applicable |
| 1 | To introduce a new medical device into the market which has a predicate device available | Traditional 510(K) |
| 2 | If a manufacturer introduces changes introduced to the device that is already existing in the market and has obtained a 510(K) clearance | Special 510(K) |
| 3 | If submission relies on FDA guidance documents voluntary consensus standard demonstration of compliance with special controls for the device type | Abbreviated 510(K) |
Step 5: The 510(K) Submission Process
Step 5.1: FORM FDA 3601 Medical Device User Fee Cover Sheet
Visit webpage User fee website to register and make payment.
Take a printed copy of this user fee cover sheet. This could be the first page of the 510(K).
Step 5.2: FORM FDA 3514 CDRH PREMARKET REVIEW SUBMISSION COVER SHEET
Download form FDA 3514 pdf. This form captures detailed information required for the different types of submissions.
A cover letter and/or the FDA Form 3514 should follow the User fee cover sheet. If FDA Form 3514 is not affixed, then the cover letter should contain all the elements relevant to the submission contained in Form 3514. This will expedite the processing time.
Step 5.3: 510(K) Submission Acknowledgement receipt by FDA
If a valid eCopy and a proper user fee has been paid Acknowledgment Letter get received from DCC through email. If the proper fee and a valid eCopy are submitted by the holder, then the holder receives an acknowledgement letter from the DCC through an email.
The following are identified by the Acknowledgement Letter:
- Receipt’s date (the date that FDA received the 510(k) submission, an eCopy and the proper user fee payment);
- The receipt’s date (this is the day that 510(k) submission was received by FDA, valid eCopy, and proper user fee payment); and 510(k) number
Step 5.4: Document Contents in a 510(K) Submission
Below is the minimum list of contents necessary to be available in 510(K) submission documents.
- Cover Letter
- Table of Contents
- Indications for Use (FDA Form 3881)
- 510(K) Summary or Statement
- Truthful and accurate statement as required by 21 CFR 807.87(l)
- Class III Summary and Certification (to be submitted when claiming equivalence to a Class III device) As per 21 CFR 807.94
- Financial Certification & Disclosure Statement
- Declaration of Conformity and Summary Reports
- Proposed Labelling
- Sterilization and Shelf Life
- Biocompatibility
- Device Specifications
- Substantial Equivalence Comparison
- Software
- Electromagnetic compatibility and Electrical Safety
- Performance Data (Summary on Clinical and Non-Clinical data)
- Additional Requirements
- Summary
Mode of Submission to FDA – E copies
In section 745A(b)(1) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 U.S.C. 379k-1) FDA is amending its regulations on medical device submissions to remove requirements for paper and multiple copies and replace them with requirements for a single submission in electronic format.
Submissions in electronic format include eCopies, submissions created and submitted on CD, DVD, or flash drive and mailed to FDA, and eSubmissions, submission package produced by an electronic submission template.
Fees, Exemptions and Waivers
Under the user fee system, medical device companies pay fees to the FDA when they register their establishments and list their devices with the agency, whenever they submit an application or a notification to market a new medical device in the U.S. and for certain other types of submissions.
The MDUFA (Medical Device User Fee) website User Fee has information on the current fee charges applicable. Payment must be received and processed at the time or before the date the application is sent.
If the FDA receives an application without full payment of all required fees, the FDA will consider the application incomplete and will not begin its review.
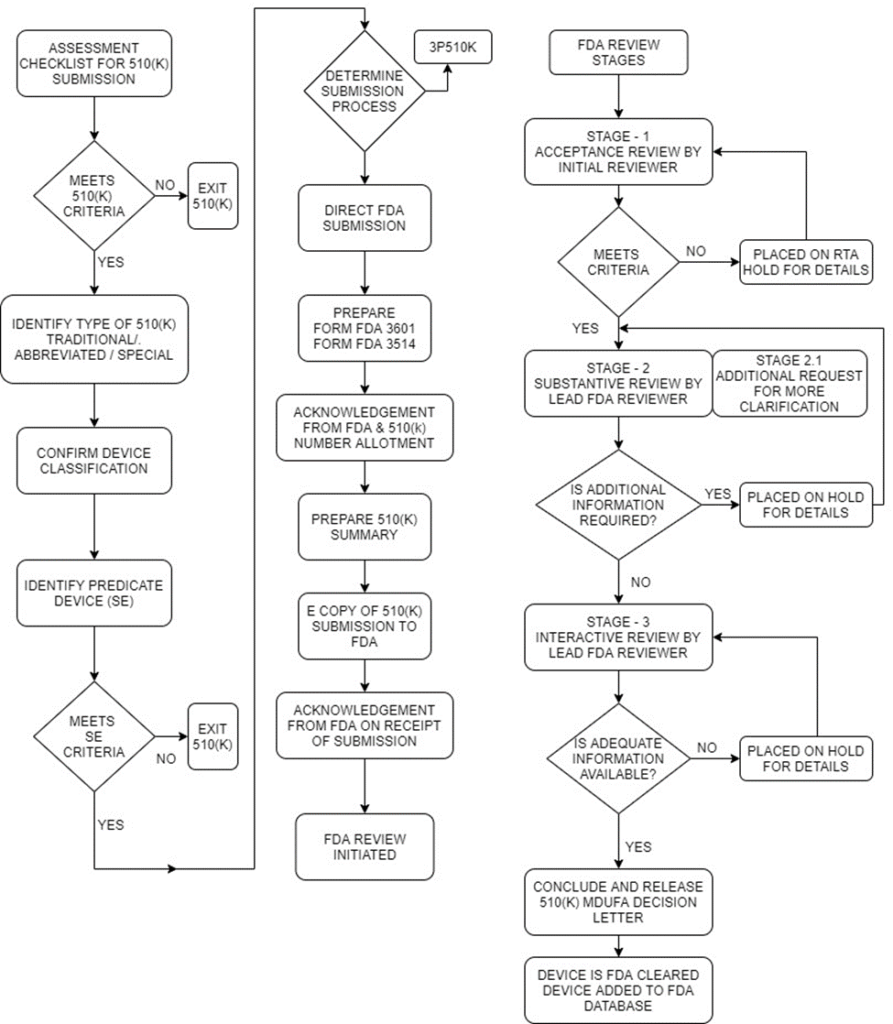
Review Stages
Acceptance Review
- This is based on the Refuse to Accept (RTA) policy by the FDA. It is a mechanism adopted by the FDA to provide a quick review of the 510(K) submission.
- This stage is only the initial (Acceptance review) stage where the FDA reviewer using separate checklists for each type of submission (traditional, abbreviated and special) reviews the submission and gives a declaration if the submission contents meet the minimum threshold requirements or is placed on RTA hold.
- The FDA reviewer evaluates the submission against specific acceptance criteria and informs the submitter within the above timeline on acceptance or indicate the missing element(s) in submission.
- In order to enhance the consistency of FDA’s acceptance decisions and to help submitters better understand the types of information FDA needs to conduct a substantive review, this guidance, includes the checklists to clarify the necessary elements and contents of a complete 510(k) submission.
Only if this stage is cleared, the submission gets qualified for the actual Substantive review stage. The reviewer conducting substantive review is the actual Lead reviewer.
Substantive Review
During Substantive Review, the Lead Reviewer conducts a comprehensive review of the 510(k) submission and communicates with the submitter through a Substantive Interaction, which should occur within 60 calendar days of receipt of the 510(k) submission.
Substantive Interaction communication is typically:
- an email stating that FDA will proceed to resolve any outstanding deficiencies via Interactive Review; or
- an Additional Information (AI) request which places the submission on hold.
Additional Information (AI) request
If the lead reviewer sends an AI request, then it means the submission is placed on hold. The submitter has 180 calendar days to address the queries from the date of additional information request by FDA reviewer.
If the queries are not addressed by the applicant within this time span, then 510(K) submission is deleted from the FDA database and the applicant will need to submit a new 510(K) to pursue the FDA market clearance process.
The submitter must submit the response, with a valid eCopy, to the DCC. The response should:
- include the submitter’s name;
- list the 510(k) number;
- identify the submission as Additional Information (AI) to the 510(k);
- list the date of FDA’s request for additional information; and
- provide the requested information in an organized manner.
Interactive Review
If the Lead Reviewer chooses to continue with an Interactive Review, this means the Lead Reviewer has determined that any outstanding deficiencies may be adequately addressed within the timeframe set by the Medical Device User Fee Amendment of 2012 (MDUFA III) performance goal for a 510(k) (90 FDA days) and that the submission will not be placed on hold.
The Lead Reviewer communicates with the submitter during the Interactive Review using tools such as:
- Telephone Call
During Interactive Review, the Lead Reviewer may request additional information from the submitter, who may either send the information to the Lead Reviewer directly or to the DCC (Document control centre).
Note: During Interactive Review, any information submitted to the DCC must include a valid eCopy.
Timeline – An overview of 510(K) Submission
Day:01
FDA receives the 510(K)-application submission
Day:07
FDA sends the acknowledgement letter (or) FDA sends HOLD letter (in case of issues)
Day:15
FDA conducts Acceptance Review and informs the applicant if the application is eligible for substantive review (or)
Places it under RTA Hold
Day:60
FDA conducts Substantiative Review and communicates on the next move towards Interactive Review
Day:90
FDA sends Final MDUFA Decision Letter
Step 6: Final 510(K) Decision Letter
MDUFA Decisions for 510(k) submissions include findings of substantially equivalent (SE) or not substantially equivalent (NSE).
When a decision is made, FDA will issue the decision letter to the submitter by email to the email address provided in the 510(k) cover letter.
- A 510(k) that receives an SE decision is considered “cleared.”
- FDA adds the cleared 510(k) to the 510(k) database, which is updated weekly.
- The IFU and the summary will be sent as attachments to the SE letter. The IFU will not be signed since it is considered an attachment to the SE letter. Therefore, the signature on the SE letter will apply to both the letter and the IFU.
If FDA does not reach an MDUFA decision within 100 FDA days (i.e., 10 days after the MDUFA goal), FDA will issue a Missed MDUFA Communication, which is written feedback to the submitter to be discussed in a meeting or teleconference, including the major outstanding review topic areas or other reasons that are preventing FDA from reaching a final decision, with an estimated date of completion.
510(K) decision-making flow chart
Disclaimer: Regulations/legislations are subjected to changes from time to time and the author claims no responsibility for the accuracy of information.
