MoH: Ministry of Public Health
MoH website: https://moph.gov.af/en
Regulatory Authority: Afghanistan Food and Drug Authority National Medicine and Health Products Regulatory Authority (NMHRA)
Link for Regulatory Authority: https://afda.gov.af/index.php/en/market-0
Afghanistan Drug Registration Procedure
Pre-Market Afghanistan Drug Registration Procedure
In the ever-changing healthcare landscape of Afghanistan, it is critical to effectively regulate medical goods and services. This paper explores the complex pre-marketing processes, including planning and policy, licensing, marketing permits, supply, price, and the vital oversight and management of clinical trials.
1. Policy & Plan
Planning and Coordination is the first step towards a strong regulatory system. The Ministry of Public Health in Afghanistan assumes a leading role in coordinating the efforts of many parties participating in the pre-marketing phase. Manufacturers, scientists, medical experts, and governmental organizations fall under this category. The foundation for efficient cooperation and communication is laid by strategic planning.
The success of pre-marketing operations depends heavily on clear and efficient communication. It is necessary to keep stakeholders updated on guidelines, regulations, and policy changes. Clear and frequent communication channels are set up to provide manufacturers, distributors, and other pertinent parties with information.
Information is a key component of regulatory decision-making, according to information management and evaluation (M&E). Strong data management systems make sure that data is gathered, examined, and assessed about clinical trials, healthcare goods, and market dynamics. Mechanisms for routine monitoring and assessment are in place to determine where improvements might be made and to gauge the success of current policies.
The foundation of healthcare regulation is the drafting and revision of legislative documents. Lawmakers in Afghanistan create and amend laws on a regular basis to meet changing needs and conform to global norms. Carefully constructed legislative documents handle licensing, permits, cost, and clinical study regulation.
2. Licensing
This essential pre-marketing step makes sure that only safe and efficient medical supplies reach consumers. A rigorous vetting process is required of businesses looking to sell their goods in Afghanistan. Complete information provided during the licensing application is used by the regulatory body to evaluate the product’s quality, safety, and efficacy.
3. Permission to Price and Supply the Market (Valuation)
Supply Chain Oversight: Businesses need permits to sell their goods to keep a safe and effective supply chain. By ensuring that products are supplied and distributed in accordance with regulatory criteria, this reduces the possibility that inferior or counterfeit goods may enter the market.
Pricing and Valuation: Accessible healthcare is facilitated by the establishment of reasonable pricing structures and the appraisal of healthcare items. To establish fair prices, regulatory agencies collaborate with manufacturers, considering aspects including quality, production costs, and market conditions. The goal of this procedure is to achieve a balance between sustainability and affordability.
4. Control and Regulation of Clinical Research
Clinical research is essential to expanding our understanding of medicine and guaranteeing the security and effectiveness of medical treatments.
To safeguard participants and maintain ethical standards, clinical research are tightly regulated and controlled by Afghan regulatory organizations. This method includes constant monitoring, informed consent processes, and a close examination of study protocols.
Post-Market Afghanistan Drug Registration Procedure
Beyond the original drug registration procedure, there is a continuous commitment to ensuring the safety, efficacy, and quality of pharmaceuticals.
1. Examining and Implementing Laws and Rules
Supervisory Authority: Afghanistan passionately believes that post-market inspections are essential to upholding pharmaceutical standards. To make sure that manufacturing sites, distribution networks, and retail locations are adhering to laws and regulations, regulatory bodies regularly audit them. The goal of this thorough inspection is to find and address any deviations from accepted norms.
Cooperation with Stakeholders: Afghan regulatory agencies work closely with pharmaceutical companies, medical practitioners, and other stakeholders to improve post-market surveillance. This cooperative strategy encourages a shared accountability for maintaining pharmaceutical standards, with continual education and communication to address issues and encourage adherence.
2. Pharmacovigilance, or Market Surveillance
Pharmacovigilance Framework: An essential part of Afghanistan’s post-market processes is market surveillance via pharmacovigilance. Data on the safety of pharmaceutical products are systematically collected, analysed, and interpreted as part of the pharmacovigilance framework. This continuous procedure aids in identifying and evaluating side effects or any other issues relating to drugs.
Adverse Event Reporting: It is crucial for patients, healthcare providers, and pharmaceutical companies to report adverse occurrences connected to their products. There is a strong reporting mechanism in place that makes it possible to promptly submit data on unanticipated side effects or other safety issues. To decide what steps to take next, regulatory bodies thoroughly look at these reports.
Risk management and signal detection: One aspect of pharmacovigilance work is the ongoing observation of signals that can point to safety issues. Regulatory agencies strive to identify new dangers and proactively address them. This could entail changing the product’s label, releasing safety alerts, or, in the worst situations, taking the medication off the market.
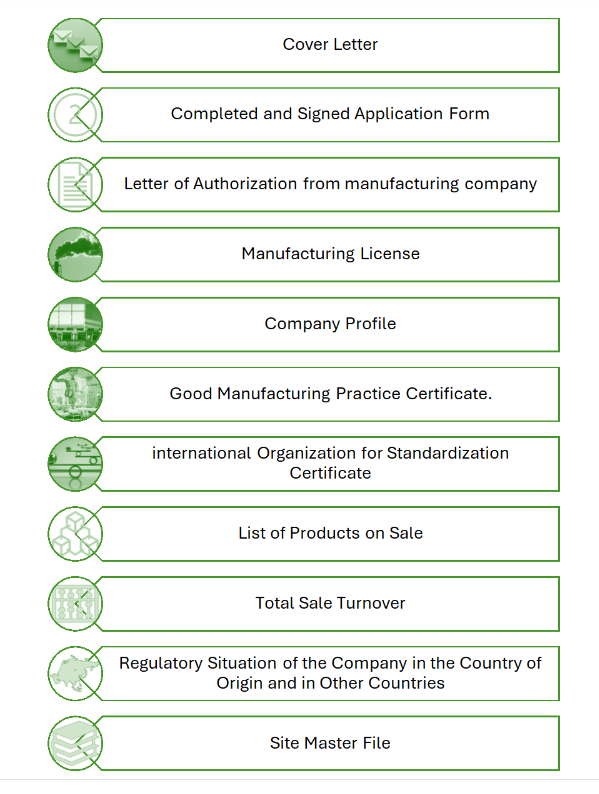
Documents Required for Product Registration in Afghanistan
1. Official introduction-letter and addresses of the company issued by the company itself.
2. License of producing medicine and medical equipment and certification of three authorized organizations of the origin country and that of WHO.
3. Special license for producing specific items from MoPH and certifications from high authorities of the origin country and that of WHO.
4. License of exportation.
5. Documents for the usage of the products inside the origin country.
6. Quality control documents of the company and also quality control documents from the central laboratory and ministry of MoPH of the origin country.
7. Specific documents of the medicine such as: list of formulation including standard formulation of the medicine, list of prices including reasonable prices, packing list with the specification about packing material, and method of packing. Procedure for controlling the production process, specification of its contents, documents of analysis, method of analysis, reliability of the method of analysis certification of the contents and pharmacological report of the medicine.
8. Original stamp of the company on the relevant documents.
9. Certification of the ministry of MoPH and stamp of chambers of commerce of the origin country and issuance of them through trading department of the embassy of Afghanistan located on the producing country.
10. Analysis the sample of the medicine in the general medicine analyzing laboratories of Afghanistan and registering the mentioned products.
Registration Requirements for Product Registration in Afghanistan
Fees for Afghanistan Drug Registration
AFN (50,000)
License Validity for Afghanistan Drug Registration
3 years.
Local Authorized Representative
Yes, a local Authorized Representative is required before you place your product on the market.