The European Union’s Medical Device Regulation (MDR 2017/745) introduces a new requirement for manufacturers producing implant cards for medical devices.
As per EU MDR, implantable devices are any devices, other than the active implantable devices, that are partially or completely implanted into the human body or are used to replace an epithelial surface or the surface of the eye, by clinical intervention, and are meant to stay in place after the procedure.
The device that is intended to be partially implanted into the human body by medical interventions and to remain in place for at least 30 days following the surgery are also considered as an implantable device.
Legal manufacturers must now provide an ‘Implant Cards(IC)’ to patients, which ensures transparency and access to device information with which they have been implanted.
Ready to Streamline Your Regulatory Compliance?
Join hundreds of companies who trust OMC Medical for their regulatory needs. Get expert guidance and ensure compliance across all markets.
Call Now +44 208 066 7260This is done per the regulations mentioned in Article 18 of the EU MDR the information provided in this article is taken from Article 18 of EU MDR and Factsheet for manufacturers of implantable medical devices .
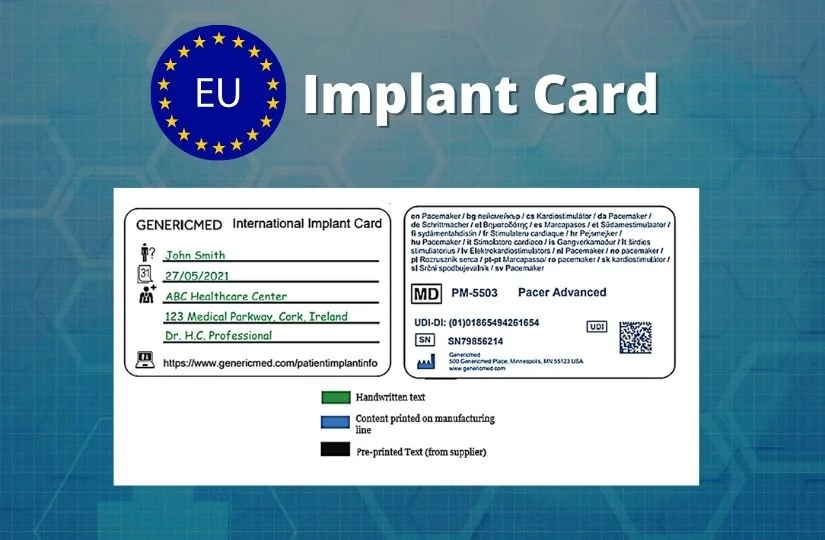
The implant cards will have the following details printed on them:
- Device name
- Device type
- UDI or unique device identification
- Serial /lot or batch number
- Name and address of the manufacturer
- Website of the manufacturer
The manufacturer should design the implant cards in such a way that it includes the following blank fields to be filled out by the implanting healthcare institution or healthcare provider:
- Name of the patient or patient ID
- Date of implantation
- Name and address of the healthcare institution that performed the implantation
The manufacturer should design the implant cards in such a way that it includes the following blank fields to be filled out by the implanting healthcare institution or healthcare provider:
- Name of the patient or patient ID
- Date of implantation
- Name and address of the healthcare institution that performed the implantation
In addition to the above, the following information should be provided to the patient bearing the implant:
- Any warnings, precautions, or measures that the patient or a healthcare practitioner should take in the event of reciprocal contact with external influences, medical exams, or environmental factors that are reasonably predictable
- Information on the device’s projected lifetime, and any necessary follow-up
- Any other information to ensure safe use of the device by the patient
- Device’s overall qualitative and quantitative information on the materials and substances to which patients can be exposed
All information shall be updated whenever required, and the updates should be conveyed to the patient via the manufacturer’s website. The basic shape of the IC shall be designed in compliance with ISO/IEC 7810 ID-1 (credit card form).
2 Example for Individual Device Implant Cards (ICs)
The symbols used already exist in the ISO standards and hence widely accepted. The following picture provides an example for individual device implant cards (ICs).

The table below provides explanation of the symbols used on an IC.MDCG 2019-8 v2 guidance for implant card (IC)