Articles
6월 뉴스레터에서 최신 업데이트와 통찰력을 공유할 수 있기를 기대합니다.안에 뭐가 들어있어요! !의료기기 규정 및 규정 준수에 관한 최신 정보화장품 안전기준 및 산업동향약물 승인 및 시장 뉴스에 대한 통찰력 확보아래에서 자세한 내용을 읽어보세요.June Newsletter Korean...
June Newsletter 2024 – Simplified Chinese
我们期待在六月时事通讯中分享最新的更新和见解。里面是什么! !有关医疗器械法规和合规性的最新信息化妆品安全标准及行业趋势深入了解药品审批和市场新闻请阅读下面的更多信息。June Newsletter Chinese (Simplified)...
June Newsletter 2024 – Chinese (Traditional)
我們期待在六月時事通訊中分享最新的更新和見解。裡面是什麼! !有關醫療器械法規和合規性的最新信息化妝品安全標準及產業趨勢深入了解藥品審批和市場新聞請閱讀下面的更多資訊。June Newsletter Chinese (Traditional)...
June Newsletter 2024
We’re excited to share the latest updates and insights in our June newsletter! What’s Inside!! Read detailed information below: https://omcmedical.com/wp-content/uploads/2024/07/June-Newsletter-2024-English.pdf...
eCTD- Electronic Common Technical Document
eCTD-an overview In today’s economy, life science companies are seeking for strategies to continue growing despite impending patent expirations, increasing generic competition, and rising drug development costs. The International Conference on Harmonization (ICH) created the Common Technical Document (CTD), which is quickly replacing other submission formats as the preferred or compulsory one by regulators in the major global markets and beyond. In Europe, Japan, and Canada, the CTD has be...
Regulatory Pathway for Clinical Investigation In the UK
Clinical investigation creates a set of clinical data that is crucial to understanding the effectiveness of a medical device. Clinical Investigation is done for devices that are previously UKCA / CE / CE UKNI marked or for research medical devices. It is governed by a clinical investigation plan with an objective, methodology, and record keeping. Steps involved in Clinical Investigation in UKCA Step 1: Ensure all the information necessary to demonstrate compliance with all the relevant esse...
Different Levels Of Labelling
The different levels of labelling are related to the different levels of packaging. There are 3 levels of packaging which corresponds to the levels of labelling. 3 Levels of Packaging corresponds to Levels of Labelling Primary Packaging (Levels of Labelling) The Primary packaging is the packaging that most closely touches a product and is often referred to as “retail packaging.” Its main goals are to ensure the protection of the product of all the medical devices and inform or...
ISO 15223-1: 2016
Many countries demand that medical devices provide textual information in their local language. When different languages are incorporated on a single label or piece of paperwork, this can cause issues with translation, design, and logistics. Users of medical devices that are labelled in a variety of languages may get confused, and delay in locating the proper terminology can occur. As a result, internationally recognised symbols with well specified definitions were established to address these i...
Importance of Human Translations vs Machine Translations
Human translation Human translations produce accurate results because it involves at least one language expert. Translations, with a human touch to them, are precise because of the in-depth knowledge that translators carry Human translations are done by native speakers of a particular language or people certified in language translations . Therefore, it is culturally diverse, nuanced and is of high quality. However, this high-quality product is expensive. Machine translation Machine transla...
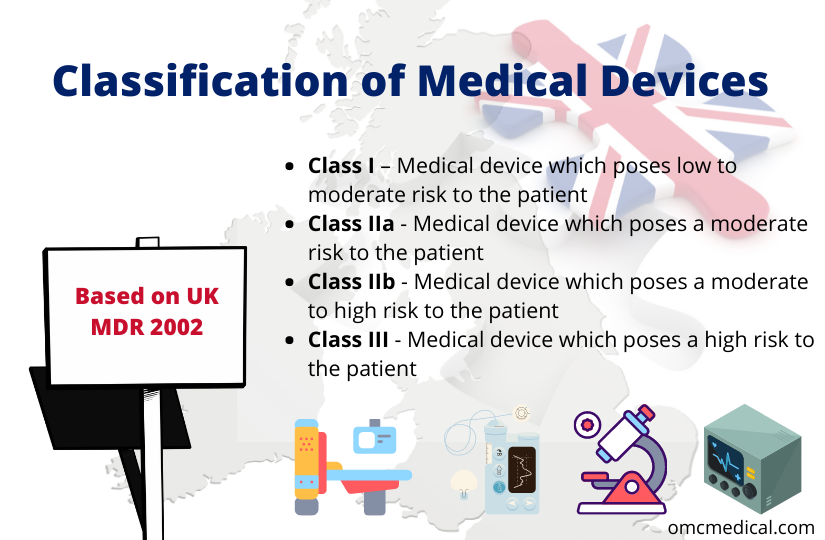
Classification of Medical Devices Based on UK MDR 2002
The classification of medical devices in the UK market will be based on UK MDR 2002 from 1st January 2021. Medical devices are classified into a particular class based on the level of risk they pose to the patient and according to the intended purpose of use. The medical devices are classified based on the classification rules in Annex IX of Directive 93/42 into one of the following four classes: The medical devices are classified into the above-given classes based on 18 rules men...