Articles
January Newsletter – EU Regulations MDCG 2022-1: Notice to 3rd country manufacturers of SARS-CoV-2 In vitro diagnostic medical devices | 12 January 2022 This notice is addressed to manufacturers of In-Vitro Diagnostic medical devices (IVDs) with the intended purpose to detect and/or quantify markers of SARS-CoV-2 infection1 who are based in countries outside the EU or the EEA and who place or intend to place the abovementioned devices on the EU market. It is intended to highlight a n...
March Newsletter
AUSTRALIA March Newsletter: Consumer/Patient information materials requirements | 02 March 2022 On 26 October 2017, the Australian government passed laws requiring implanted and active implantable medical devices to include patient information materials (patient information booklets and patient implant cards). More flexibility in information delivery was introduced by providing electronic formats. Manufacturers of all implanted devices in the ARTG (Australian Register of Therapeutic Goods),...
April Newsletter
AUSTRALIA Transitioning to the new Therapeutic Goods Advertising Code | 05 April 2022 The Therapeutic Goods Administration (TGA) is updating advertisers on the transitional arrangements for the 2021 Advertising Code, particularly regarding hard copy ad stock. On January 1, 2022, the Therapeutic Goods (Therapeutic Goods Advertising Code) Instrument 2021 (2021 Code) went into effect. The Therapeutic Goods Advertising Code (No. 2) 2018 is repealed and replaced by the 2021 Code (2018 Code). Advertis...
May Newsletter 2022
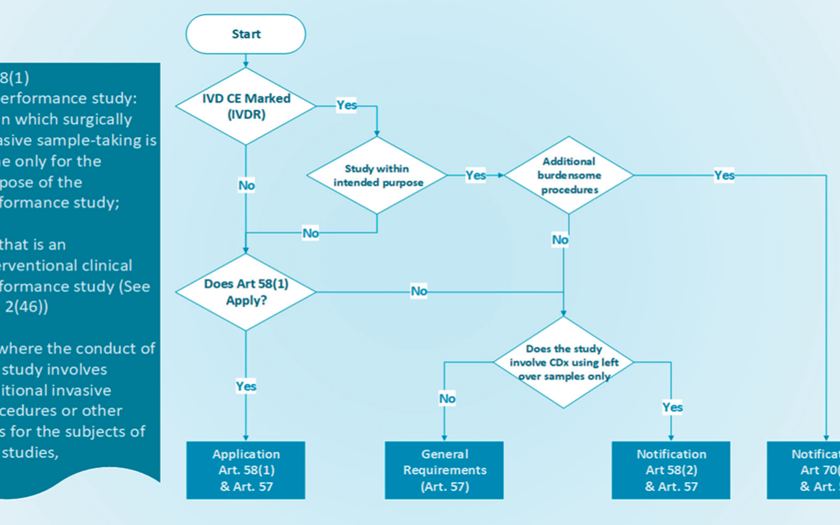
EUROPEAN UNION (EU) Stricter rules for placing medical tests on the market | 25 May 2022 From 26 May 2022, new laws for in vitro diagnostic medical devices (IVDR) such as HIV testing, pregnancy tests, and COVID-19 tests are in effect. The guidelines will improve public health and patient safety for these devices, putting EU law in line with technological advancements and medical research progress. It also ensures equitable market access for manufacturers by unifying market access standards acros...
June Newsletter
EUROPEAN UNION (EU) – JUNE NEWSLETTER Guidance document on the implementation of EU Product Rules 2022 | 29 June 2022 The ‘Blue Guide’ on EU product rules 2022 (Document Number (2022/C 247/01). This June newsletter guidance discusses both non-food and non-agricultural products, referred to as products for use by consumers or professionals. It applies to medical and in-vitro diagnostic devices that comply with Regulation (EU) 2017/745 and Regulation (EU) 2017/746. It covers various topi...
July Newsletter 2022
EUROPEAN UNION (EU) Harmonised administrative practices and alternative technical solutions| 13 July 2022 The MDCG guidance document advises the Member States and other relevant parties on applying specific IVDR regulations in the absence of Eudamed. This guidance defines harmonised administrative processes and alternative technology alternatives for information interchange until Eudamed is entirely operational. Based on the appropriate provisions of Directive 98/79/EC, this guidance covers spec...
August Newsletter 2022
EUROPEAN UNION (EU) MDCG guidance on Notified body capacity and availability of medical devices and IVDs |26 August 2022 The MDCG acknowledges that there are still significant and urgent challenges to be overcome to ensure that manufacturers are prepared and that notified bodies have enough capacity to certify medical devices and in vitro diagnostic medical devices following the EU MDR and the IVDR within the transition periods specified in the Regulations. According to the information acquired...
September Newsletter 2022
EUROPEAN UNION (EU) Notified Bodies Position paper on application of hybrid audits for QMS assessments under MDR and IVDR|26 September 2022 The latest position paper discusses the need for hybrid audits instead of the traditional on-site audits which were previously followed. The document represents the collective position on aspects to be considered while employing Information and Communication Technologies (ICT)-based auditing for quality management systems. Manual on borderline medical device...
October Newsletter 2022
Guidance on EU Authorized Representatives | 31 October 2022 Medical Device Coordination Group has updated its guidance on Authorized Representatives under MDR 2017/745 and IVDR 2017/746. Under both MDR and IVDR, it is a requirement for manufacturers based out of the European Union to appoint an Authorized representative. The MDCG 2022-16 document further includes the following topics: Guidance on Requirements relating to notified bodies | 27 October 2022 The requirements for notified bodies unde...
November Newsletter
EUROPEAN UNION (EU) EU guidance on reference laboratories for IVD medical devices | 30 November 2022 EU Commission has published new guidance for candidate reference laboratories in the In vitro diagnostic medical devices field. The document guides laboratories planning to apply in any of the EU Member states. The key topics included are as follows: Notified Bodies under MDR and IVDR | 21 November 2022 The 35th Notified Body, SGS Fimko Ltd, for Regulation (EU) 2017/745 on Medical Devices (MDR),...