| Symbol | Usage |
Manufacturer
 | This symbol is used to represent the manufacturer and should be accompanied by manufacturer’s name and address adjacent to the symbol. |
Country of Manufacturer  | To identify the country of manufacturer, only used when there is a separate above information is not provided in the labelling. CC standard for the two-letter country code. The country code is defined by ISO 3166. |
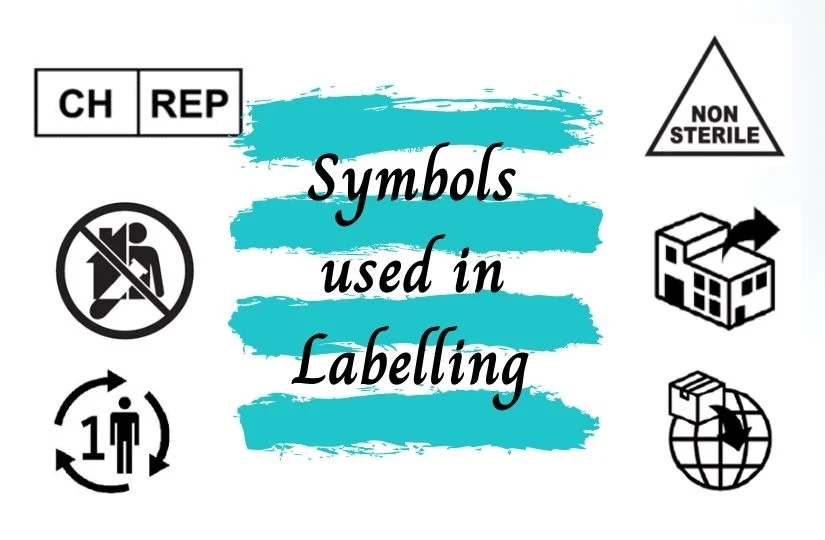
Authorised Representative
 | The Authorised representative (AR) in Europe is specifically identified using this symbol and should be accompanied by the Authorised representatives name and address adjacent to the symbol. If the AR is the identified as the other responsible entities name and address need not be duplicated. *Approved by European union and not part of the ISO standard. |
 | The Authorised representative (AR) in Switzerland is specifically identified using this symbol and should be accompanied by the Authorised representatives name and address adjacent to the symbol. If the AR is the identified as the other responsible entities name and address need not be duplicated. *Approved by Swiss Medic and not part of the ISO standard. |
Importer
 | The Importer in specifically identified using this symbol and should be accompanied by the Importer’s name and address adjacent to the symbol. If the Importer is the identified as the other responsible entities name and address need not be duplicated. |
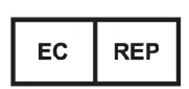
Distributor
 | The Distributor is specifically identified using this symbol and should be accompanied by the Distributors name and address adjacent to the symbol. If the Distributor is the identified as the other responsible entities name and address need not be duplicated. |
Translation
 | Must indicate that the medical device label has been translated by someone other than the device manufacturer. This symbol has been prepared to reduce the need for multiple translations of words into national languages, simplify and rationalise the labelling of medical devices, thereby reducing risk of misidentification, promoting safety for the patient and reducing the amount of training required by health care personnel. *In most cases this is required to be identified if the translation is provided by a local entity who doesn’t have proper qualification as a professional translator, By which the information provided is only a supplement and not an approved information verified by the manufacturer. |
Medical Device
 | Indicates that the device is a Medical Device |
Invitro Device
 | Indicates the device is an Invitro device |
Sterilization

| When the device is sterilized by using vaporised hydrogen peroxide this symbol should be used. |
Sterilization  | When the device has been provided sterile and is sterilized by using aseptic process techniques. |
Sterilization  | When the device has been provided sterile |
Sterilization  | When the device is sterilized by using ethylene oxide |
Sterilization  | When the device is sterilized by using irradiation |
Sterilization  | When the device is sterilized by steam or dry heat |
Sterilization  | When a presence of a sterile fluid path within the medical device when other parts of the medical device are not necessarily supplied sterile. |
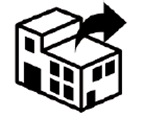
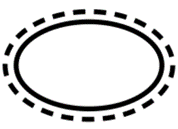 | Indicate that there is a single sterile barrier system with protective packaging outside. |
 | Indicate that there is a single sterile barrier system with protective packaging inside. |
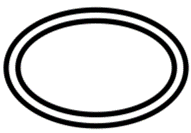 | Indicate that there are two sterile barrier systems. |
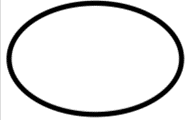 | Indicate that there are single sterile barrier systems. |
 | Indicate that the device that is normally provided sterile in the same or similar packaging has not been sterilized. |
Batch Code  | Identify the manufacturer’s batch or lot code, for example on a medical device or the corresponding packaging. The code shall be placed adjacent to the symbol. |
Serial Number  | Identify the manufacturer’s Serial number on a medical device or packaging the code shall be placed adjacent to the symbol. |
Single Patient – Multiple use
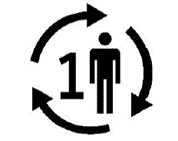 | Indicates that the medical device may be used multiple times (multiple procedures) on a single patient. |
Repackaging 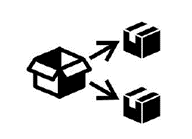 | To identify that a modification to the original medical device packaging configuration has occurred. |
 | Unique Device Identification is a symbol used to identify the device identification number accompanied by the number adjacent to the device. In some cases bar codes, QR codes will be shown instead of the number. |
| PATIENT CARD or IMPLANT CARD symbols according to the EU IVDR EU 2017/746 regulation |
 | Health care centre or doctor – This is to indicate which heath care centre or doctor performed the implantation. |
 | Patient Identification: Details of the Patient |
 | Date of Implantation, the date on which the implant was placed to be identified adjacent to the symbol |
 | Patient information website, the website link where the patient can identify the details about the implant placed within them should be indicated here. |
| Symbols used for IVDR according to the EU IVDR EU 2017/746 regulation |
 | For self-testing If the IVD can be used for self-testing by the end user. |
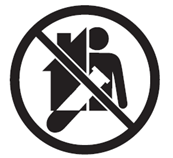 | Not for Self-testing |
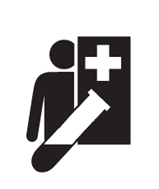 | Near Patient testing If the IVD should only be used near patient setting by a healthcare professional. |
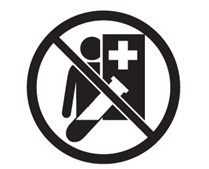 | Not for Patient testing If the IVD should only be used by a healthcare professional or a laboratory professional. |