If you are planning to supply medical devices in Australia, understanding the Therapeutic Goods Administration (TGA) medical device registration costs and timelines is essential for effective market entry.
In 2025, the TGA continues to apply a risk-based approach to device regulation, meaning costs and approval timelines vary depending on the classification of your device, your evidence of conformity, and whether you choose the TGA Conformity Assessment or Mutual Recognition pathway.
At OMC Medical Limited, we help manufacturers, importers, and distributors navigate the Australian Register of Therapeutic Goods (ARTG) requirements, ensuring faster approvals, cost efficiency, and full compliance with Australian regulations.
Medical Device Registration Costs & Timelines in Australia
Let’s get started,
TGA Medical Device Registration Costs
The TGA charges two main types of fees for medical device registration:
- Application Fees – Paid when submitting your ARTG application.
- Annual Charges – Ongoing fees for keeping your device listed in the ARTG.
Example fee structure for 2025:
| Fee Type | Official Cost (AUD, 2025) | Notes |
|---|---|---|
| Application Fee | $621 (Class I), $1,187 (Class IIa/IIb), $1,530 (Class III) | Based on device class |
| Conformity Assessment Fee | $4,700 (Level 1 audit, minimum) – $17,288+ (Level 2/Class III) | Higher for Class III and AIMDs; varies based on audit complexity |
| Annual ARTG Listing Fee | $114 (Class I other), $828 (Class Is/Im), $1,230 (Class IIa/IIb), $1,566 (Class III/AIMD) | Paid yearly |
💡 Tip: Costs can increase for complex devices requiring additional assessment. Choosing the right approval pathway can reduce costs significantly.
TGA Medical Device Registration Timelines
Approval timelines vary depending on the pathway and device risk class:
⏳ Why timelines vary: Missing documentation, incorrect classification, or complex clinical evidence requirements can extend TGA review times. OMC ensures all documentation is right the first time to avoid delays.
Factors That Affect Costs & Timeline
Several elements influence how much you will spend and how long approval will take:
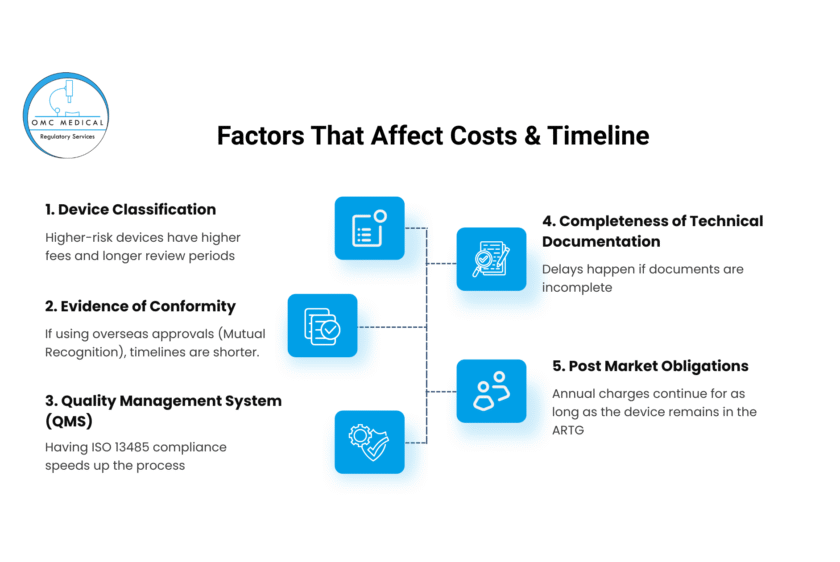
- Device Classification – Higher-risk devices have higher fees and longer review periods.
- Evidence of Conformity – If using overseas approvals (Mutual Recognition), timelines are shorter.
- Quality Management System (QMS) – Having ISO 13485 compliance speeds up the process.
- Completeness of Technical Documentation – Delays happen if documents are incomplete.
- Post-Market Obligations – Annual charges continue for as long as the device remains in the ARTG.
Example: A Startup Launching a Home Blood Pressure Monitor
Emma runs a small health-tech startup in the UK. Her company developed a Class IIa home blood pressure monitor that already had CE Mark approval in Europe.
Her challenge:
She wanted to sell in Australia but was concerned about costs and approval delays.
What happened:
- Because her device was already CE certified, Emma’s team qualified for the Mutual Recognition pathway with the TGA.
- They reused their existing CE technical documentation — saving time on preparation.
- The TGA reviewed and approved her device in just under 3 months, instead of the typical 9–12 months for a full conformity assessment.
The result:
Emma saved thousands of dollars in assessment fees and got her product to Australian customers before her competitors.
How OMC Medical Can Help You Save Time & Costs
At OMC Medical Limited, we:
- Assess your device classification to choose the most cost-effective pathway.
- Prepare & review your technical documentation to meet TGA requirements.
- Act as your Australian Sponsor if you are an overseas manufacturer.
- Manage the entire TGA submission process, reducing costly delays.
- Ensure ongoing compliance with post-market surveillance and vigilance reporting.
With years of experience in medical device registration in Australia, our team ensures you meet TGA regulatory requirements quickly and cost-effectively.
Conclusion
The costs and timelines for registering a medical device in Australia depend on multiple factors — from your device’s risk class to your chosen approval pathway.
By understanding the TGA fee structure and processing times, you can plan a smoother market entry strategy for 2025.
📩 Contact OMC Medical Limited today to discuss your device, get an accurate cost & timeline estimate, and streamline your TGA medical device registration process in Australia.