As India improves its rules for medical devices, it’s important to know how products are grouped when they are registered. The Central Drugs Standard Control Organization (CDSCO) has created clear guidelines for grouping medical devices. This helps make the submission process easier, avoids repeating work, and makes the rules clearer. Grouping allows manufacturers to organize similar products better, making the approval process smoother and more predictable.
What is Medical Device Grouping in India?
Medical device grouping in India refers to the process of categorizing medical devices with similar features, intended use, or technology so they can be registered under a single license application with the Central Drugs Standard Control Organization (CDSCO). This approach is designed to make the registration and licensing process simpler, faster, and more cost-effective for manufacturers, importers, and distributors.
Grouping allows applicants to submit one application for a set of related devices, instead of applying separately for each product. This reduces paperwork, saves time, and lowers costs during the approval process.
6 Types of Medical Device Grouping
CDSCO has specified several categories for grouping medical devices:
Ready to Streamline Your Regulatory Compliance?
Join hundreds of companies who trust OMC Medical for their regulatory needs. Get expert guidance and ensure compliance across all markets.
Call Now +44 208 066 7260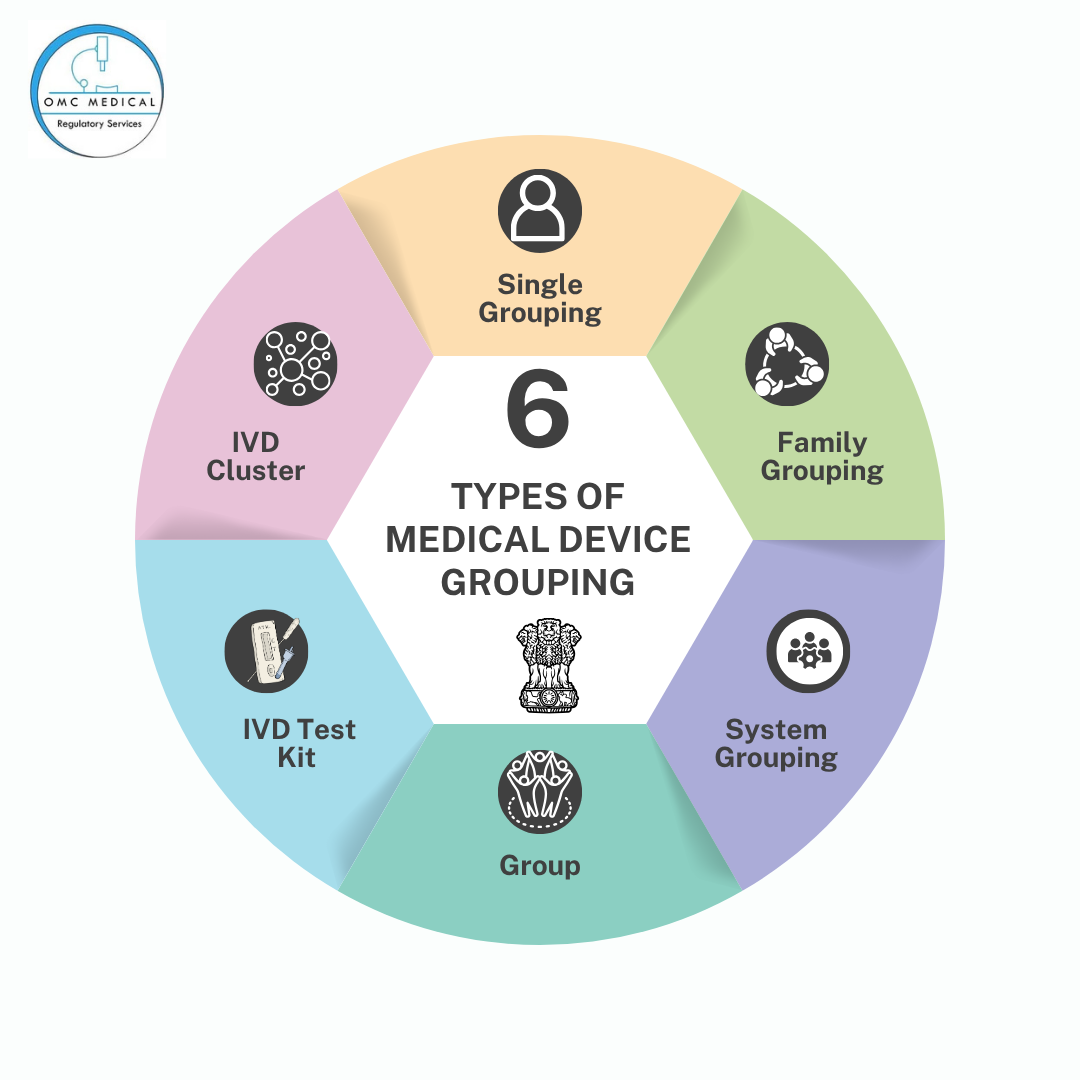
- Single Grouping: For individual devices that are packaged and sold separately. Each device in this group requires its own license and documentation.
- Family Grouping: Devices that have the same intended use, design, manufacturing process, and risk classification, but may differ in size, shape, or minor features. These can be grouped under a single license.
- System Grouping: Devices that are designed to work together as a system for a common purpose (e.g., a blood glucose monitoring system with meter, test strips, and lancets).
- Group: A collection of devices that are sold together as a kit but may not necessarily be designed to function as a system.
- IVD Test Kit : An IVD test kit comprises multiple components or reagents packaged together for a specific diagnostic purpose, such as an HIV rapid diagnostic kit.
- IVD Cluster: This grouping is specific to in vitro diagnostics and includes devices that are technologically similar, intended for the same condition or analyte, but sold as separate products (e.g., individual reagents for a specific assay).
Key Factors of Grouping
According to the CDSCO guidelines, devices can be grouped based on factors such as:
- Same Manufacturer: Devices must come from the same legal manufacturer.
- Same Risk Classification: Grouping is allowed when devices share the same risk class (A, B, C, or D) according to the Medical Device Rules (MDR) 2017.
- Common Intended Purpose: Devices with a similar intended use or indication can be grouped.
- Same Design and Manufacturing Process: Devices that share the same technological features and manufacturing methods qualify.
- Same Proprietary Name or Brand: Devices marketed under a common brand name may be grouped.
Benefits of Grouping for Manufacturers
- Simplified Regulatory Submissions: Reduces the number of individual applications required.
- Faster Review Timelines: Streamlined dossiers for related devices allow for quicker regulatory processing.
- Cost Efficiency: Grouping minimizes documentation and regulatory fees.
- Harmonization with Global Standards: CDSCO’s grouping is aligned with international practices, promoting smoother market entry for global manufacturers.
How OMC Can Help
OMC Medical makes it easy for you to register your medical devices in India. We guide you through every step-helping you group your products correctly, prepare the right documents, and communicate with CDSCO. Our experts save you time, reduce costs, and make sure your registration process is smooth and stress-free.
Check it out –
Conclusion
The CDSCO Medical Device Grouping Structure serves as a strategic tool for medical device manufacturers and importers navigating the Indian regulatory landscape. By following these guidelines, stakeholders can optimize regulatory pathways, reduce time to market, and ensure compliance with India’s evolving medical device regulations.
OMC Medical supports companies in making the most of these grouping guidelines by providing expert guidance, helping you select the right grouping strategy, prepare necessary documentation, and streamline your CDSCO registration process for faster and smoother market access.







