Overview & Key Authority
The regulatory process in Bolivia is overseen by the National Authority of Medicines and Health Technologies (Agencia Nacional de Medicamentos y Tecnologías en Salud – AGEMED), which operates under the Ministry of Health and Sports.
The process is structured but can be unpredictable. A typical timeline can range from 12 to 24 months, heavily dependent on the device classification, the quality of the submitted dossier, and AGEMED’s workload.
Bolivia Medical Device Registration Step-by-Step Process and Estimated Timeline
Here is a breakdown of the stages involved:
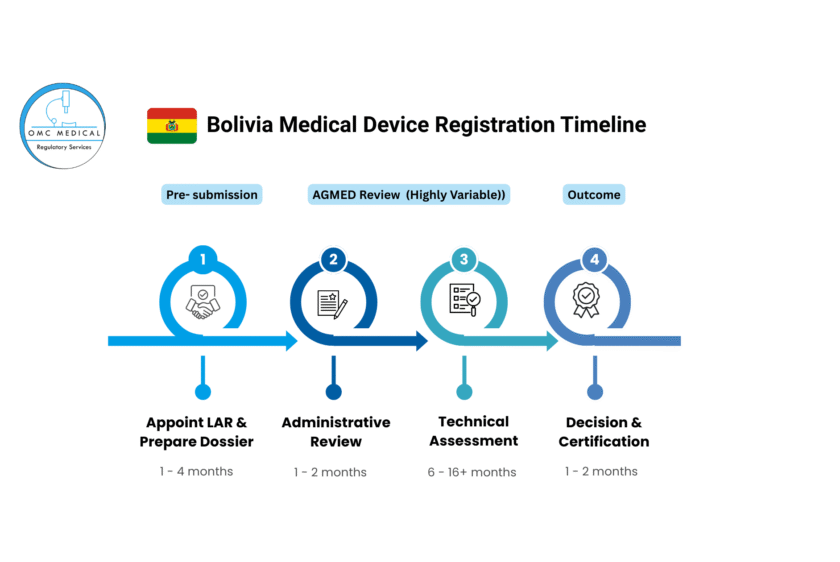
Phase 1: Pre-Submission Preparation (1-4 Months)
This is the most variable phase, depending on your readiness.
- Step 1: Appoint a Local Authorized Representative (LAR): Mandatory. Foreign manufacturers must have a legal entity in Bolivia to act as their registrant and point of contact with AGEMED. This is the first critical step.
- Step 2: Compile the Technical Dossier: This involves gathering all required documentation. Key requirements include:
- Free Sale Certificate (FSC/Certificate of Foreign Government): Legalized/Apostilled and translated.
- Certificate to Foreign Government (CFG) or ISO 13485 Certificate: Often required, must be legalized and translated.
- Quality Management System (QMS) certificates.
- Product information: Labels, IFUs, technical specifications, biocompatibility, stability studies, etc. All must be translated into Spanish.
- Evidence of marketing approval in country of origin (e.g., FDA 510(k)/PMA, CE Certificate, etc.).
- Step 3: Product Classification: Determine your device’s risk class (Class I, II, III, or IV) based on Bolivian regulations, which are broadly aligned with other Latin American systems.
Phase 2: Submission and AGEMED Review (8-18+ Months)
This is the official regulatory review phase.
- Step 4: Dossier Submission: Your LAR submits the complete application to AGEMED.
- Step 5: Administrative Review (1-2 months): AGEMED checks the application for completeness. If documents are missing, the clock stops until the LAR provides them.
- Step 6: Technical/Scientific Review (6-16+ months): This is the longest phase. AGEMED reviewers evaluate the safety, efficacy, and quality of the device. They will likely raise questions or requests for clarification (“Requests for Information” – RFIs). The speed of your response directly impacts the timeline.
- Step 7: Decision: Upon successful review, AGEMED issues the Sanitary Registration Certificate (Certificado de Registro Sanitario).
Phase 3: Post-Approval
- Step 8: Maintenance: Registrations are typically valid for 5 years and can be renewed. Any significant changes to the device, label, or manufacturing process may require a regulatory submission (variation) to AGEMED.
Visual Timeline Chart
The following chart illustrates the typical timeline and its potential variability:
Critical Factors Influencing the Timeline
- Device Classification: Class I devices are generally faster than Class III/IV devices.
- Dossier Quality: A complete, well-organized, and perfectly translated dossier significantly reduces the risk of delays from RFIs.
- AGEMED’s Workload: Processing times can fluctuate based on the agency’s backlog and resources.
- Response Time to RFIs: How quickly you and your LAR respond to AGEMED’s questions is crucial.
- Legalization & Translation: The process of getting documents apostilled and professionally translated takes time and must be done correctly.
Recommendations
- Engage a Local Expert: Using an experienced Local Authorized Representative (LAR) or regulatory consultant in Bolivia is not just mandatory; it is the most important factor for success. They understand the nuances of the process and can navigate AGEMED efficiently.
- Start Early: Begin document preparation long before you plan to launch.
- Validate Documents: Ensure all foreign certificates are apostilled/legalized and that all translations are accurate.
- Plan for Delays: Build buffer time into your market entry plan. Do not assume the process will be fast.
Disclaimer: This information is for guidance purposes only. Regulations and processes can change. Always consult with your Local Authorized Representative in Bolivia or legal counsel for the most current and specific advice for your device.







