This article provides a comprehensive overview of the mandatory medical device registration process with the National Center for Health Technology and Equipment (Centro Nacional de Tecnologías y Equipos en Salud – CNTES), the regulatory authority operating under the Ministry of Popular Power for Health (MPPS) in Venezuela.
The Venezuelan regulatory landscape is complex and characterized by procedural volatility and extended timelines. OMC Medical emphasizes that engagement with a knowledgeable Local Authorized Representative (LAR) is not merely advised but is a de facto requirement for foreign manufacturers. A typical registration process can take 18 to 36 months, subject to significant variation.
Regulatory Authority
- Authority: National Center for Health Technology and Equipment (Centro Nacional de Tecnologías y Equipos en Salud – CNTES)
- Affiliation: Ministry of Popular Power for Health (MPPS).
- Regulatory Framework: Based on the Ley de Medicamentos (Drug Law) and resolutions by the Gaceta Oficial, though guidance is often updated via internal circulars.
Key Prerequisite: Local Authorized Representative (LAR)
Foreign manufacturers must appoint a legally established entity in Venezuela to act as their LAR. The LAR is the official registrant and sole point of contact with CNTES, bearing full legal responsibility for the product in the Venezuelan market.
OMC Medical’s Role: We leverage our network of trusted, experienced LAR partners in Venezuela to act on your behalf, ensuring compliance and navigating the administrative complexities.
Step-by-Step Registration Process of Venezuela
Phase 1: Pre-Submission Preparation (3-6 Months)
- Step 1: LAR Appointment & Power of Attorney (PoA): Formalize the relationship with your LAR via a PoA. This document must be notarized, apostilled (or legalized by a Venezuelan consulate), and translated into Spanish.
- Step 2: Dossier Compilation: Prepare the technical and administrative documentation. Key requirements include:
- Free Sale Certificate (FSC): Issued by the health authority of the country of origin (e.g., FDA, EMA, PMDA, Health Canada). Must be apostilled/legalized and translated.
- Certificate of Foreign Government (CFG): For US manufacturers, this is often required alongside the FSC.
- ISO 13485 Certificate: Proof of Quality Management System compliance.
- Certificate of Approval/Marketing Authorization: Evidence of approval in the country of origin (e.g., FDA 510(k)/PMA Letter, CE Certificate).
- Product Information: Complete technical file, including intended use, specifications, labels, Instructions for Use (IFU), and material safety data. All must be in Spanish.
- Evidence of Stability/Shell Life: Where applicable.
- Authorized Representative Agreement.
Phase 2: CNTES Submission & Review (12-30+ Months)
- Step 3: Initial Submission: The LAR submits the complete dossier to CNTES. An initial filing fee is paid.
- Step 4: Administrative Review (1-3 Months): CNTES checks the application for completeness. Incomplete dossiers are rejected, restarting the clock.
- Step 5: Technical/Scientific Assessment (10-27+ Months): This is the most prolonged and unpredictable phase. CNTES experts review the dossier for safety, quality, and efficacy. They will issue questions or Requests for Information (RFIs). The speed of your response is critical.
- Step 6: Decision & Fee Payment: Upon successful review, CNTES issues an approval resolution. The LAR must pay the final registration fee to secure the certificate.
Phase 3: Post-Approval
- Step 7: Sanitary Registration Grant: CNTES issues the Sanitary Registration Certificate (Certificado de Registro Sanitario).
- Step 8: Renewals: Registrations are typically valid for 5 years and require a renewal application submitted well before the expiration date.
- Step 9: Variations: Any significant change to the device, manufacturing process, or label requires a pre-approval submission to CNTES.
Estimated Timeline
The following chart provides a visual estimate of the highly variable timeline:
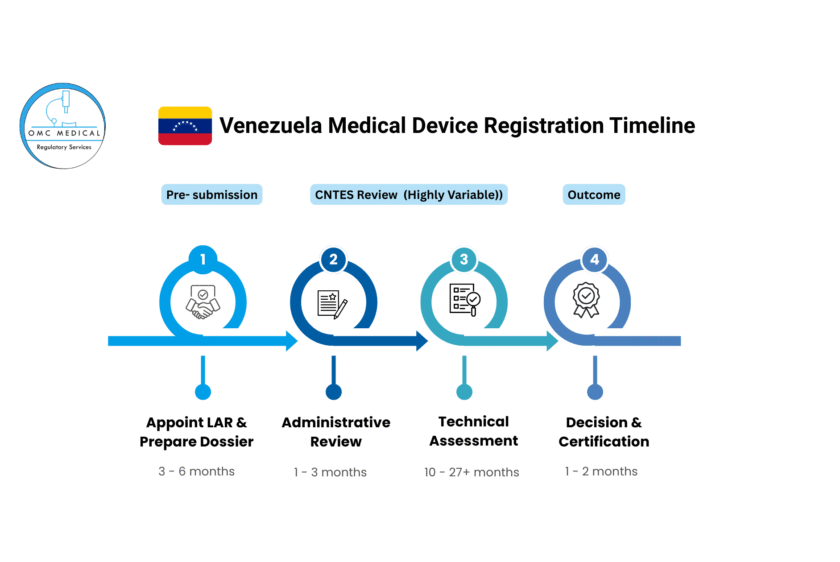
Note: This timeline is an estimate. Periods of political or economic instability can lead to complete halts in CNTES’s operational capacity, potentially extending the process beyond 36 months.
OMC Medical’s Value Proposition
Navigating the Venezuelan market alone is high-risk. Partnering with OMC Medical provides:
- Expert LAR Liaison: We manage the relationship with your LAR, ensuring clear communication and accountability.
- Dossier Preparation & Gap Analysis: We ensure your technical file meets CNTES’s specific, often unwritten, requirements to avoid rejection.
- Document Management: We manage the complex process of apostille, consular legalization, and professional translation.
- Application Monitoring: We proactively track your application’s status and manage RFI responses to minimize delays.
- Strategic Guidance: We provide realistic timelines and risk assessments to inform your commercial planning.
Conclusion
Entering the Venezuelan market requires significant patience, resources, and local expertise. The process is among the most challenging in the Latin American region. By engaging OMC Medical, you mitigate these risks through a structured, expert-led approach.
We recommend initiating conversations with our consultants 24-36 months before your desired market entry date.







