Zambia
Zambia
Safeguarding Public Health Through Comprehensive Regulation
MoH: Zambian Ministry of Health
MoH Website: https://www.moh.gov.zm/
Regulatory Agency: The Zambia Medicines Regulatory Authority
Regulatory Agency Website: https://www.zamra.co.zm/
Regulation: Pharmaceutical Act No 14 of 2004 and Medicines and Allied Substances Act No. 3 of 2013 of the Laws of Zambia
Fees: ZMK 759,060
Local Authorised Representative: Yes
License Validity: 5 years
Overview
- The Authority’s primary goal is to make sure that all medications and related substances supplied to Zambians continuously fulfill the established requirements for efficacy, safety, and quality.
- The Republic of Zambia’s Ministry of Health oversees the Pharmacy and Poisons Board, which oversees handling drug and pharmaceutical registrations in Zambia.
- The Pharmaceutical Act requirements, subject to various restrictions, that any medication that is to be marketed in Zambia must be covered by a product license or marketing authorization that has been granted by the Authority.
- The Pharmacy and Poisons Act, Chapter 299 of the Laws of Zambia, was originally passed in 1941, marking the beginning of Zambia’s history with respect to pharmaceutical control.
- This Act regulated commerce in pharmaceuticals and poisons and the pharmacy profession. This statute established the Pharmacy and Poisons Board (PPB) to supervise its implementation.
Requirements for registration of a medicine
- Applicant required to submit a dossier in CTD format.
- The registration of a patent is not a requirement for a pharmaceutical product to be marketed.
- The applicant is required to submit an application, a sample(s) and dossiers(s) for each product which should contain the following information:-
(a) the name and address of the applicant in full
(b) the name and address of the medicine
(c) the dosage form of the medicine
(d) the active constituents of the medicine
(e) the indications of the medicines and method of use
(f) the centra-indications, warnings and precautions of the medicine
(g) the composition (ingredients of the medicine and the reasons for their inclusion of if non-active as well as their specifications such as BP, USP etc)
(h) the shelf-life (this should be supported bv stabilitv studies data)
(i) the containers and packaging (should give full description i.e technical specifications)
(j) the labeling should be part of the dossier and should contain the following information:
the name of the medicine
– the pharmacological properties
– the names and quantities of active ingredients
– the quantity of medicines i.e. 100ml, 1000 tablets etc.
– the directions for use
– the contra-indications, warnings and precautions
– the storage instructions
– the manufacture date
– the batch number
– the expiry date
– the license number
– the name and address of the manufacturer
– the method of sale, e.g. General sale, Pharmacy sale only or Prescription sale only.
(k) the distributor’s name and address
(l) an original copy of the World Health Organization (WHO) pharmaceutical certificate of quality and free sale certificate addressed specifically to Zambia
(m) the name and designation of the person signing the application
(n) where the medicine is to be imported for Zambia for the first time:-
– the chemistry of the medicine
– the pharmacological data
– the toxicological data
– the teratology
– the clinical studies
– the countries in which the sale of the medicine has been authorized.
(o) the final product specifications and certificate of analysis.
(p) the certificates of analysis of all the raw materials used.
Application for marketing authorisation of a medicine for human use
Module1: Administrative documents
1.0. Cover letter
1.1. Comprehensive review
1.2. Application Information
1.2.1. Application Form
1.2.2. Letter of authorisation for communication on behalf of the applicant
1.2.3. Electronic copy declaration
1.2.4. Copy of certificate for a Vaccine Antigen Master File (VAMF)
1.2.5. Copy of certificate for a Plasma Master File (PMF)
1.2.6. Copy of certificate(s) of suitability of the European Pharmacopoeia (CEP)
1.2.7. Confirmation of Prequalification (CPQ) of an API
1.2.8. Letter of access from the APIMF holder, CEP holder or CPQ holder.
1.3. Labelling and packaging
1.3.1. Package Insert/SmPC
1.3.2. Summary of product characteristics (SmPC)
1.3.3. Patient Information Leaflet
1.3.4. Label
1.3.5. Braille
1.4. Information about the experts
1.4.1. Declaration signed by the expert – Quality
Information about the Expert – Quality
1.4.2. Declaration signed by the expert – non-clinical
Information about the Expert – Non-clinical
1.4.3. Declaration signed by the expert – Clinical
Information about the Expert – Clinical
1.5. Specific requirements for different types of applications.
1.6. Enviornmental risk factors
1.7. Good manufacturing practices
1.7.1. Date of last inspection of each site
1.7.2. Inspection reports or equivalent document
1.7.3. Latest GMP certificate or a copy of the appropriate license
1.7.4. Registration of Responsible Pharmacist or Suitably Qualified Person, for local manufacturer.
1.7.5. Certified copy of permit to manufacture specified controlled substances.
1.8. Details of screening. (Screening checklist)
1.9. Individual patient data- Statement of availability.
1.10. Foreign regulatory status
1.10.1. List of countries in which an application for the same product as being applied for has been submitted, approved, rejected or withdrawn
1.10.2. WHO type Certificate of a Pharmaceutical Product
1.10.3. Registration certificates or marketing authorisation
1.10.4. Foreign prescribing and patient information
1.10.5. Data set similarities
1.11. Regional Summaries
1.11.1. Summary of Bioequivalence Studies
1.11.1.1 Study Title(s) (or brief description giving design, duration, and subject population of each study)
1.11.1.2. Protocol and study numbers.
1.11.1.3. Investigational products (test and reference) detalls in tabulated format, including
active ingredient, strength, dosage form, manufacturer, batch no, expiry or retest date, country in which procured.
1.11.1.4. Confirmation that the test product formulation and manufacturing process is that being applied for
1.11.1.5. Name and address of the Research Organisation(s) / Contract Research Organisation(s)
where the bioequivalence studies were conducted
1.11.1.6. Sponsor and responsible sponsor representative: name and address, contact details
1.11.1.7. Duration of Clinical phase: dates of dosing and last clinical procedure
1.11.1.8. Date of final report
1.11.2. Biostudy reference product confirmation
1.11.3. Certificates of analysis of the test and reference products
1.11.4. Bioequivalence trial information form (BTIF)
1.11.5. Biowaiver requests in relation to conducting comparative bioavailability study Quality
1.11.6. Information Summary (QS)
1.12. Pediatric development program (reference to Pediatric development program)
1.13. Information relating to pharmacovigilance
1.13.1 Pharmacovigilance system
1.13.2 Risk Management System
1.14 Electronic review documents (e.g. product information, BTIF, ZAMRA-QOS)
1.15 Sample and Documents (e.g. FPP, device(s), certificates of analysis)
1.15.1 Confirmation of submission of a sample
1.15.2 CoA of the sample
Module 2: Summary of the dossier
2.1. Table of contents of module 2
2.2. Introduction
2.3. Quality overall summary
Module 3: quality
Module 4: Non- Clinical study report
Module 5: clinical study report
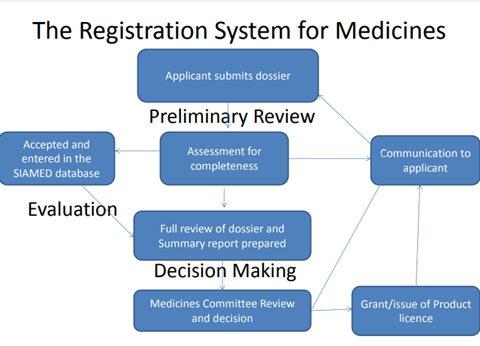
Review Process
- Preliminary Review
- Application completeness.
- Application must be in the CTD format and supported by detailed technical documentation as may be appropriate.
- Administrative information on the manufacturing site, manufacturer status, fees paid, and samples for the applicant (Potential PLH).
- Acknowledgment and registration in PRIMS.
- Evaluation
- This depends on the nature of the application- Standard application -Fast track.
- Timelines for evaluation are yet to be established.
- The nature of the product—that is, whether it is produced locally, WHO-approved, registered by strict DRAs both inside and outside the region, a new chemical entity, or a generic application—determines the extent of the evaluation.
- Due to insufficient capacities QC is not routinely carried out as part of evaluation.
- GMP inspections may be performed on the suggestion of the Medicines Committee.
- The assessment report is written in the appropriate format and submitted to the Medicines Committee together with the Q, S, and E recommendations.
- The Committee may direct additional submission of the necessary information, in which case its decision may be favorable, negative, or postponed.
- If the answer is yes, a product license is granted, subject to any applicable restrictions.
- The applicant must pay the yearly retention required by the Act and adhere to the labeling requirements outlined in the rules.
- If the Medicines Committee issues a negative decision, the applicant is informed accordingly, and reasons given in writing for non-issuance of the product license.
- The applicant may file an appeal in accordance with the procedures for appeals against the Authority’s decisions.
- Should further information be needed, the applicant is asked to provide it within a certain amount of time; otherwise, the application may be rejected.
- A WHO computer-assisted medicines registration application known as SIAMED is used to manage data regarding a registered product.
- There is a process in place for modifications, and the Authority must be notified of any changes.
Classification of Medicine
Herbal Medicine
Herbal medicinal products are defined as pharmaceuticals having one or more herbal preparations, one or more herbal substances, or one or more of these substances combined with one or more herbal preparations as active components.
Homeopathic Medicine
In compliance with the provisions of Directive 2004/27/EC, Member States that recognize their tradition of homeopathic practice may apply specific regulations to homeopathic medicines, which are unique forms of medicinal products. The Medicinal Products (Control of Placing on the Market) Regulations (S.I. 540 of 2007), which were created under the Irish Medicines Board Acts of 1995 and 2006, give this Directive legal force in Ireland.
Drug Approval Process
- Centralized Process- A business in the European Union (EU) may apply to the European Medicines Agency (EMA) through the centralized procedure for a single marketing authorization that is accepted by all EU members as well as Iceland, Liechtenstein, and Norway. It is required for several types of medications to employ this licensing system, such as:
- medications with novel active ingredients used to treat specific ailments (AIDS, cancer, diabetes, and others)
- medications for advanced therapy
- pharmaceuticals produced using biotechnology pharmaceuticals
- uncommon human illnesses (sometimes referred to as “orphan medicines”)
Quick Contact
If you have any questions or need help, feel free to contact with our team.
©2024 OMC Medical, All Rights Reserved. With Love by 7oroof.com

Our team will be happy to respond your queries. Contact us directly with your questions or for scheduling FREE consultation and we’ll be in touch as soon as possible.
Quick Contact
If you have any questions or need help, feel free to contact with our team.

Our team will be happy to respond your queries. Contact us directly with your questions or for scheduling FREE consultation and we’ll be in touch as soon as possible.
Quick contact
- info@omcmedical.co.uk
-
0044 7719761764
0044 2080667260 - Planet House, North Heath Lane, Horsham, West Sussex RH12 5QE

Our team will be happy to respond your queries. Contact us directly with your questions or for scheduling FREE consultation and we’ll be in touch as soon as possible.
Our Branches
- Switzerland
- Europe (Northern Ireland)
- Asia
- Canada
- Brazil
- Middle East
- China
- Turkey (Partner Office)

To launch a medical device in a country, medical devices must comply with the local country’s regulatory requirements. Let us be your trusted partner in bringing your medical devices to the Global market. Contact us today to learn more about how we can assist you in every step of the way.
Our Branches
- Europe
- Asia
- Africa
- Oceania
- South America
- North America
- Planet House, North Heath Lane, Horsham, West Sussex RH12 5QE
- Planet House, North Heath Lane, Horsham, West Sussex RH12 5QE


