South Korea Cosmetic Product Registration
South Korea Cosmetic Product Registration
COSMETIC REGISTRATION IN SOUTH KOREA
Cosmetics fall under two categories: functional cosmetics and regular cosmetics. MFDS reviews only functional cosmetics for pre-market approval. For all other regular cosmetics, the MFDS authorized the Korea Pharmaceutical Traders Association (KPTA) to review and certify import permission requests submitted by the Korean importer.
Regulatory Authority
Ministry of Food and Drug Safety (MFDS)
Link for Regulatory Authority
Local Regulation
Cosmetics Act and its Enforcement Decree
Who can Register?
- Manufacturers and importers (domestic or foreign)
- Appointing a local RP is mandatory to represent the manufacturer/importer and manage regulatory compliance within South Korea.
Data to be Communicated
- Information verifying safety, effectiveness, or functionality.
- Data regarding the origin and specifics of research and development.
- Information regarding safety data.
- Data confirming effectiveness or functionality.
- Data related to Sun Protection Factor (SPF), waterproof Sun Protection Factor (SPF), and UV-A Protection Factor (PA).
- Information about standards and testing methodologies (including samples).
Process to Register Cosmetic Product
Regarding functional cosmetics, any Responsible Seller planning to manufacture or sell such products through manufacturing or importing must undergo evaluation by the Minister of the Ministry of Food and Drug Safety (MFDS). Alternatively, they must submit a safety and effectiveness report for each product to the Minister of the MFDS, as outlined in Article 4 of the Cosmetics Act (Examination, etc. of Functional Cosmetics). This requirement also extends to any revisions made to previously evaluated aspects.
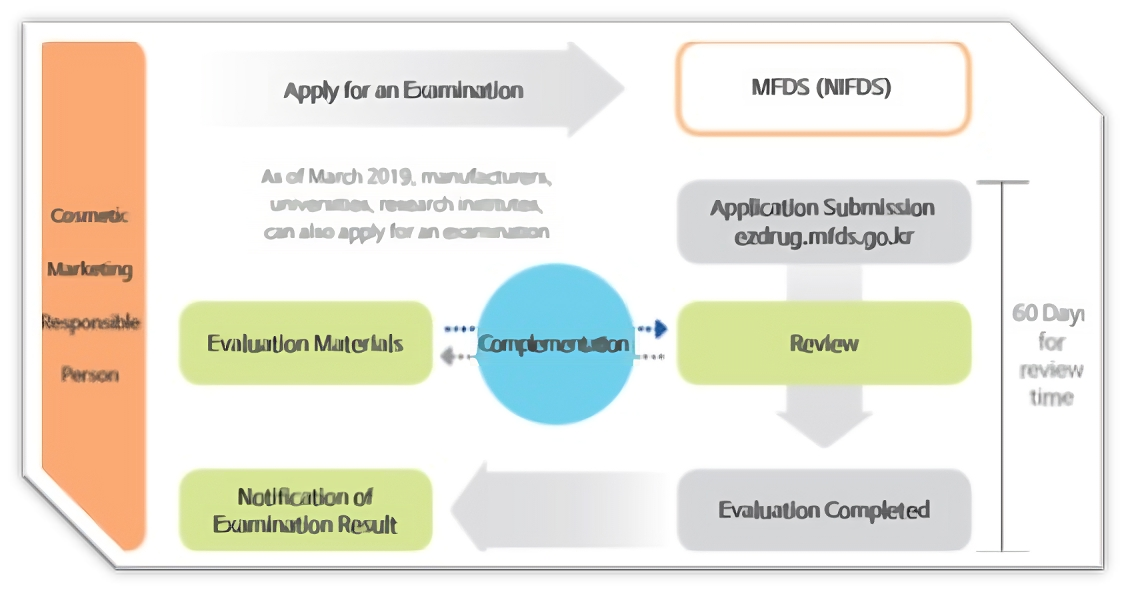
Certain ingredients have already received approval from the MFDS for use in specific types of functional cosmetics, albeit with restrictions. In such cases, if a manufacturer or importer intends to market a functional cosmetic containing only these approved ingredients, they are not required to provide clinical or efficacy data. The approval for these products can be obtained within approximately 7 days through a submission procedure.
Conversely, if the manufacturer/importer seeks to utilize unapproved ingredients, they must submit clinical and efficacy data, along with identification of the active ingredients, when seeking approval from the MFDS. This approval process may take 4-6 months to complete.
Timeframe and Fees
- The approval process can take several months, depending on the product complexity and review time.
- Fees: The fees vary based on the product category and complexity of testing required. It can range from ₩100,000 to several million Korean won (hundreds to thousands of USD).
Additional Information
- Labelling: All cosmetic products, regardless of category, must comply with specific labelling requirements in Korean language.
- According to the Korean Cosmetics Act, quality testing must be conducted by lot/batch. However, the testing required for products differs based on the category of the cosmetic product.
- Korean cosmetic firms are participating in the “Less Plastic” initiative by adopting biodegradable materials and minimizing the use of plastic packaging.
- Furthermore, South Korea enacted the Recycling Act in December 2019, which applies to and affects all products sold in the country, including cosmetics.
- General cosmetics are subject to a post-market surveillance and can be manufactured/imported without registration.
- The MFDS operates on a negative list system, maintaining a catalogue of “prohibited ingredients.” This list functions as a reference, permitting the use of all ingredients except those explicitly listed as prohibited. However, for preservatives, UV filters, and colorants, only ingredients authorized by the MFDS are permitted for use.
Quick Contact
If you have any questions or need help, feel free to contact with our team.
©2024 OMC Medical, All Rights Reserved. With Love by 7oroof.com

Our team will be happy to respond your queries. Contact us directly with your questions or for scheduling FREE consultation and we’ll be in touch as soon as possible.
Quick Contact
If you have any questions or need help, feel free to contact with our team.

Our team will be happy to respond your queries. Contact us directly with your questions or for scheduling FREE consultation and we’ll be in touch as soon as possible.
Quick contact
- info@omcmedical.co.uk
-
0044 7719761764
0044 2080667260 - Planet House, North Heath Lane, Horsham, West Sussex RH12 5QE

Our team will be happy to respond your queries. Contact us directly with your questions or for scheduling FREE consultation and we’ll be in touch as soon as possible.
Our Branches
- Switzerland
- Europe (Northern Ireland)
- Asia
- Canada
- Brazil
- Middle East
- China
- Turkey (Partner Office)

To launch a medical device in a country, medical devices must comply with the local country’s regulatory requirements. Let us be your trusted partner in bringing your medical devices to the Global market. Contact us today to learn more about how we can assist you in every step of the way.
Our Branches
- Europe
- Asia
- Africa
- Oceania
- South America
- North America
- Planet House, North Heath Lane, Horsham, West Sussex RH12 5QE
- Planet House, North Heath Lane, Horsham, West Sussex RH12 5QE


